Figure 1. Neurocircuits linked to withdrawal identified using pharmacologic probes.
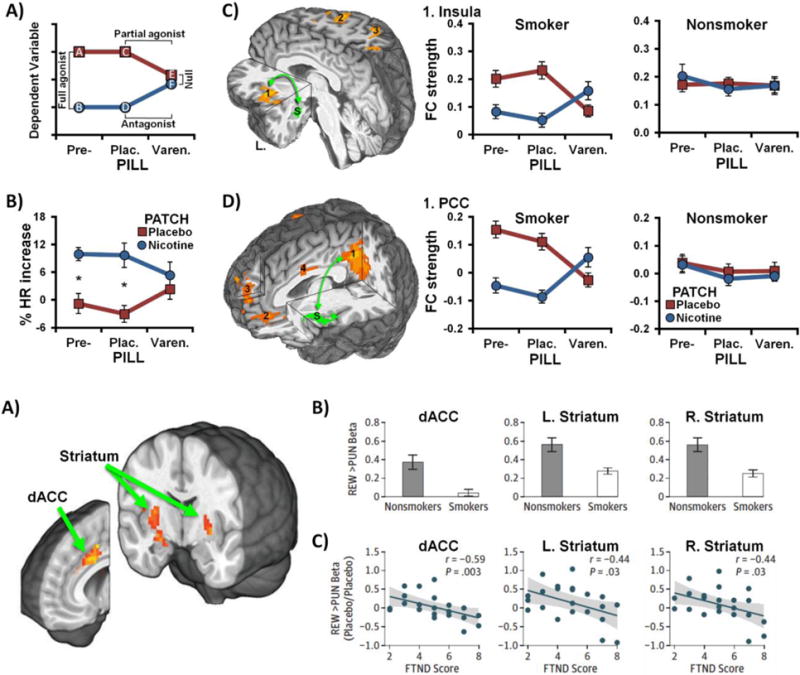
(A) Schematic of the hypothesized varenicline (pill) and nicotine (patch) pharmacologic interaction (adapted from: [22]). Withdrawal-related effects on the dependent variable were anticipated to be greatest in the absence of drug administration (placebo conditions, data points A & C). Nicotine was expected to reduce withdrawal-related elevations (B & D), consistent with a full agonist effect (A vs. B, C vs. D). Varenicline was also expected to reduce the dependent variable (E), consistent with a partial agonist effect (C vs. E). Combined varenicline and nicotine administration (F) was anticipated to produce an attenuated nicotine-induced response, consistent with an antagonist effect (D vs. F). These partial agonist and antagonist effects then yield a null effect of nicotine versus placebo patch (E vs. F) in the presence of varenicline. (B) Impact of drugs on heart rate (HR) among abstinent cigarette smokers (* p < 0.05, adapted from: [22]). (C) Amygdala-insula resting-state functional connectivity (rsFC) (left) was reduced by varenicline and nicotine in a manner consistent with the hypothesized pharmacologic interaction among abstinent smokers (middle), but not nonsmokers (right) (adapted from: [24]). (D) Insula’s rsFC with the posterior cingulate cortex (1, left) and medial prefrontal cortex (2 & 3) was similarly reduced by varenicline and nicotine among smokers (middle), but not nonsmokers (right) [24]. S: seed region (green), Pre-: before beginning study pill administration; Plac.: under placebo pills; Varen.: under active varenicline pills.
