Abstract.
Over the past 20 years, Thelazia callipaeda (the oriental eyeworm) has become endemic in Europe, infecting domestic and wild carnivores and humans. The vector of this nematode, the fruit fly Phortica variegata, has recently been discovered in the United States, and its vector competence is demonstrated for T. callipaeda in this article, therefore representing a potential new threat for infection of carnivores and humans in the United States.
Amongst vector-borne helminths, the eyeworm Thelazia callipaeda (Spirurida, Thelaziidae) is considered as an emergent zoonotic agent spreading in several European countries.1 This nematode lives in the orbital cavities and associated ocular tissues of domestic (dogs and cats) and wild (e.g., foxes, wolves, beech martens, and wild cats) carnivores and lagomorphs causing ocular disease of public health concern, because of its zoonotic potential.2 Named as “oriental eyeworm” for its distribution in Far Eastern regions, T. callipaeda has been until recently described in dogs, cats, and foxes from Italy3 and then increasingly reported in both animals and humans from several other countries from western to eastern Europe.4,5 Such a spreading of T. callipaeda throughout Europe was largely predicted by an ecological niche model6 based on data of the ecology and the seasonal occurrence of its vector, the fruit fly Phortica variegata (Diptera, Drosophilidae, Steganinae), in a highly endemic area of southern Italy. Therefore, data on the distribution, biology, and ecology of this drosophilid, both under laboratory7 and natural conditions,8 have been pivotal for understanding the risk of the introduction of the parasite into a previously nonendemic area.9
The only Thelazia species so far described in carnivores and humans from the United States is Thelazia californiensis, which is confined to California in the western United States.10 Information about the vector of this eyeworm species is limited, and both Fannia canicularis and Fannia benjamini (Diptera, Fanniidae), the little house flies, have been implicated in its transmission.11 Nonetheless, P. variegata has recently been discovered at some sites in New York in the eastern United States,12 but no data are available about its competence as intermediate hosts of T. californiensis or T. callipaeda.
Here, we infected P. variegata flies captured in Rochester, New York, with T. callipaeda nematodes collected from a dog in southern Italy, to demonstrate their competence as intermediate hosts of the oriental eyeworm.
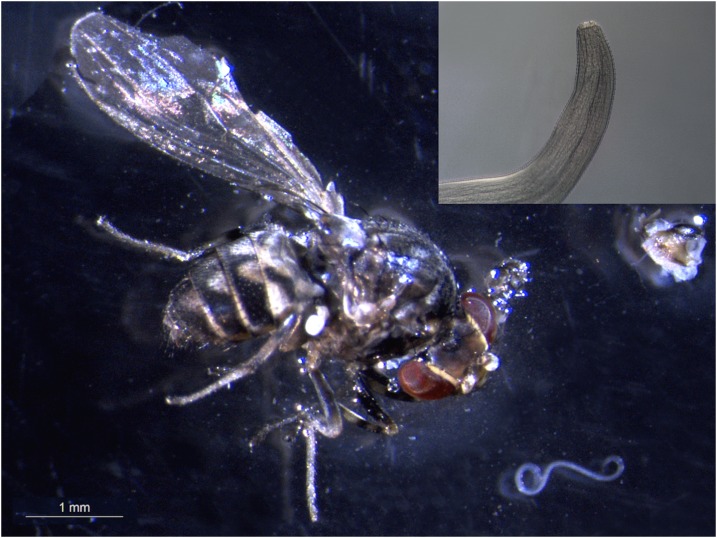
Flies were collected by J. J. in a mature hardwood forest, consisting primarily of oaks (Quercus spp.) in Highland Park, Rochester, New York (43.133°N, 77.612°W, 240 m a.s.l.). Collections were made by walking slowly through the forest and netting Phortica spp. around the collector’s face. Once captured, flies were identified as P. variegata,13 being characterized by multiple large dark spots on the scutum, wings hyaline with two interruptions along the costal vein, legs with brown coxa, and yellow tibia with three dark rings (Figure 1). After preliminary identification, 62 wild-caught individuals of P. variegata (37 males and 25 females) were placed in vials containing the sugar–agar medium and sent to the Department of Veterinary Medicine, University of Bari, Italy. Briefly, T. callipaeda specimens were collected from the eyes of a naturally infected dog in the Basilicata region, and the first-stage larvae (L1) were recovered from adult mature females by slitting the uterus into a drop of saline solution. Infection procedures were described in Otranto et al.7
Figure 1.
Morphological key characters of Phortica variegata: orbita with pale stripe (A), legs with brown coxa, dark femur (base and apex yellow), yellow tibia with three dark rings, and yellow tarsus (B), cross-veins shaded, the discal and second basal cells separated by the additional cross-vein (C) and the costal vein with two interruptions (D). This figure appears in color at www.ajtmh.org.
Fifty-four P. variegata flies were experimentally infected by L1, and the remaining eight were used as control. Forty-four flies died at different time points. Dead flies (n. 38) were stored in 70% ethanol in individual vials for molecular detection of T. callipaeda partial (689 bp) cytochrome c oxidase subunit 1 (cox1), as described.14 The remaining 10 were dissected at 21 dpi in a drop of saline solution to recover T. callipaeda larvae, resulting one female infected by L3 in the proboscis (Figure 2) and molecularly processed (Table 1). Of the 48 specimens processed by polymerase chain reaction (PCR), 20 (41.7%) scored positive at different time points (Table 1). Overall, the positive rate of flies at both dissection and molecular detection was 37%. The noninfected P. variegata scored negative to T. callipaeda at dissection and PCR. The L3 isolated at the dissection of the fly at 21 dpi measured 2.1 mm in length and 77.70 μm in width at a medium body (Figure 2), with blunt cephalic and tapering caudal ends. Cuticle with delicate transversally striated, particularly at the anterior end (Figure 2A). Buccal opening was almost rounded, with the buccal capsule resembling a vase-shaped, 9.40 μm deep, and 10.15 μm wide rest of digestive tract. The nerve ring was located at the level of middle of the esophagus (not clearly), approximately 20.0 μm from the cephalic end. The larvae showed a well-developed digestive tract.
Figure 2.
Third-stage larva of Thelazia callipaeda at the dissection of the proboscis of Phortica variegata on day 21 dpi in the square box—a detail at higher magnification of the anterior part of the larvae with a delicate transversally striated cuticle and buccal opening bearing a buccal capsule resembling a vase-shaped and a well-developed digestive tract. This figure appears in color at www.ajtmh.org.
Table 1.
Number and percentage of Phortica variegata (males, M and females, F) positive for Thelazia callipaeda in the molecular analysis at different days after infection
| Molecular assay | ||||||
|---|---|---|---|---|---|---|
| Number examined | Number infected | |||||
| Days after infection | M | F | Total | M (%) | F (%) | Total (%) |
| 1 | 2 | 3 | 5 | 2 (100) | 2 (66.7) | 4 (80) |
| 3 | 1 | 1 | 2 | 1 (100) | 1 (100) | 2 (100) |
| 6 | 13 | 7 | 20 | 6 (46.1) | 4 (57.1) | 10 (50) |
| 7 | 4 | 3 | 7 | 2 (50) | 1 (33.3) | 3 (42.8) |
| 19 | 3 | 1 | 4 | – | – | – |
| 21 | 7 | 3 | 10 | – | 1 (33.3) | 1 (10) |
| Total | 30 | 18 | 48 | 11 (36.7) | 9 (50) | 20 (41.7) |
In addition, 14/48 (29.2%) flies were molecularly processed through amplicons sequencing to confirm the morphological identification.15 Affiliation of all processed flies to P. variegata was assessed molecularly (99% nucleotide genetic identity with GenBank AN = EF576934). The study of genitalia of one male specimen by J.M. also confirmed this determination.
Data indicate that T. callipaeda from Europe may develop in the population of P. variegata flies collected in the United States, therefore representing a potential threat for infection of carnivores and humans in areas where this fly is present. Based on their relationships to known Thelazia hosts in Europe,2 potential hosts in New York State include coyotes, red fox, gray fox, black bear, raccoon, mink, least weasel, striped skunk, cottontail rabbit, and snowshoe hare.16 Therefore, the role of wildlife (e.g., foxes, jackal, hares, and wolves)2,17,18 as reservoirs of T. callipaeda should be assessed, considering that the infection most likely establishes in wildlife before spreading to dogs and other domestic animals.2 In Europe, eyeworm infection is often reported in foxes, most likely because of the more frequent exposure of wild carnivores to Phortica spp. flies.9 Also, the absence of genetic variation among nematodes collected from different hosts (i.e., dogs, cats, foxes, wolves, beech martens, wild cats, lynxes, jackals, and humans) in Europe might indicate that the same zoonotic T. callipaeda circulates among different animal species examined, including humans, and reinforces their tight association with its vector P. variegata.6 However, because T. callipaeda has been firstly isolated in Asia, further studies are required to assess the origin of its vector in the United States from Europe or from Asia.
Interestingly, an infective L3 T. callipaeda was recovered in a P. variegata female fly at 21 dpi, and 41.7% of flies (i.e., 20/48) scored positive for T. callipaeda at the molecular examination at different time points (i.e., 1, 3, 6, 7, and 21 dpi). This percentage of positivity was higher than that recorded in Otranto et al.7 (i.e., 18.4%). Although the presence of T. callipaeda DNA alone cannot demonstrate the vector role of P. variegata, it clearly indicates that the DNA of T. callipaeda is present in the flies at detectable levels. Although male flies are considered the vector in natural conditions, T. callipaeda may likewise develop both in males and females in experimental settings, and both males and females were attracted to the collector’s face in this study.7 The nematode DNA detected at different time points (i.e., from 1 to 21 dpi) matches with the timing of the developmental stages in Phortica flies, as described.7 In particular, most of the dissected flies (80–100%) scored positive to T. callipaeda DNA within 3 dpi (Table 1), suggesting that a high percentage of L1 were ingested during the experimental feeding. At 6–7 dpi, the presence of larvae DNA in 50% of flies may be related to the molted L2, which finally develop into the infective stage (L3) at 21 dpi, when only one fly scored positive at both the molecular diagnosis and at dissection. Although information on the distribution of P. variegata in the United States is sparse, data here presented suggest that the American population of P. variegata is susceptible for T. callipaeda infection, and this geographic area might be potentially suitable for the spreading of eyeworms, thus requiring an awareness of veterinarians, ophthalmologists, and medical doctors. Under the above circumstances, an ecological niche model for P. variegata distribution would be useful to predict future trends for the presence of this infection, similarly to what has been assessed in Europe.6 Importantly, the occurrence of eyeworm infections in California should be carefully assessed in consideration of the number of the reported cases in dogs (https://wagwalking.com/condition/eyeworm-thelazia-californiensis). In the meantime, the potential role of P. variegata as vectors of T. californiensis would require further investigation in relationship to data available on Fannia spp.
REFERENCES
- 1.Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petricì D, Genchi C, Capelli G, 2013. Vector-borne helminths of dogs and humans in Europe. Parasit Vectors 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otranto D, Dantas-Torres F, Mallia E, DiGeronimo PM, Brianti E, Testini G, Traversa D, Lia RP, 2009. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: report of new host species and ecological implications. Vet Parasitol 166: 262–267. [DOI] [PubMed] [Google Scholar]
- 3.Otranto D, Ferroglio E, Lia RP, Traversa D, Rossi L, 2003. Current status and epidemiological observations of Thelazia callipaeda (Spirurida, Thelaziidae) in dogs, cats and foxes in Italy: a “coincidence” or a parasitic disease of the Old Continent? Vet Parasitol 116: 315–325. [DOI] [PubMed] [Google Scholar]
- 4.Miró G, Montoya A, Hernández L, Dado D, Vázquez MV, Benito M, Villagrasa M, Brianti E, Otranto D, 2011. Thelazia callipaeda: infection in dogs: a new parasite for Spain. Parasit Vectors 4: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colella V, Kirkova Z, Fok É, Mihalca AD, Tasić-Otašević S, Hodžić A, Dantas-Torres F, Otranto D, 2016. Increase in eyeworm infections in eastern Europe. Emerg Infect Dis 22: 1513–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otranto D, Brianti E, Cantacessi C, Lia RP, Máca J, 2006. The zoophilic fruit fly Phortica variegata: morphology, ecology and biological niche. Med Vet Entomol 20: 358–364. [DOI] [PubMed] [Google Scholar]
- 7.Otranto D, Lia RP, Cantacessi C, Testini G, Troccoli A, Shen JL, Wang ZX, 2005. Nematode biology and larval development of Thelazia callipaeda (Spirurida, Thelaziidae) in the drosophilid intermediate host in Europe and China. Parasitology 131: 847–855. [DOI] [PubMed] [Google Scholar]
- 8.Otranto D, Cantacessi C, Testini G, Lia RP, 2006. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int J Parasitol 36: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 9.Máca J, Otranto D, 2014. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasit Vectors 7: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doezie AM, Kucius RW, Aldeen W, Hale D, Sith DR, Mamalis N, 1996. Thelazia californiensis conjuntival infestation. Ophthalmic Surg Lasers 27: 716–719. [PubMed] [Google Scholar]
- 11.Burnett HS, Wagner ED, 1958. Two new definitive hosts for the eye worm, Thelazia californiensis Price, 1930. J Parasitol 44: 502. [Google Scholar]
- 12.Werner T, Jaenike J, 2017. Drosophilids of the Midwest and Northeast, 2017 New York, NY: River Campus Libraries. Available at: http://humanities.lib.rochester.edu/drosophilaguide/. [Google Scholar]
- 13.Bächli G, Vilela CR, Escher SA, Saura A, 2005. The Drosophilidae (Diptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica, Vol. 39. Leiden, The Netherlands: Brill, 362 pp. [Google Scholar]
- 14.Otranto D, Testini G, De Luca F, Hu M, Shamsi S, Gasser RB, 2005. Analysis of genetic variability within Thelazia callipaeda (Nematoda: Thelazioidea) from Europe and Asia by sequencing and mutation scanning of the mitochondrial cytochrome c oxidase subunit 1 gene. Mol Cell Probes 19: 306–313. [DOI] [PubMed] [Google Scholar]
- 15.Otranto D, Stevens JR, Testini G, Cantacessi C, Máca J, 2008. Molecular characterization and phylogenesis of Steganinae (Diptera, Drosophilidae) inferred by the mitochondrial cytochrome c oxidase subunit 1. Med Vet Entomol 22: 37–47. [DOI] [PubMed] [Google Scholar]
- 16.New York State Department of Environmental Conservation , 2010. Checklist of Amphibians, Reptiles, Birds and Mammals of New York State. Albany, NY: Wildlife Diversity Group. [Google Scholar]
- 17.Hodžić A, Latrofa MS, Annoscia G, Alić A, Beck R, Lia RP, Dantas-Torres F, Otranto D, 2014. The spread of zoonotic Thelazia callipaeda in the Balkan area. Parasit Vectors 7: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihalca AD, Ionică AM, D’Amico G, Daskalaki AA, Deak G, Matei IA, Şimonca V, Iordache D, Modrý D, Gherman CM, 2016. Thelazia callipaeda in wild carnivores from Romania: new host and geographical records. Parasit Vectors 9: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]