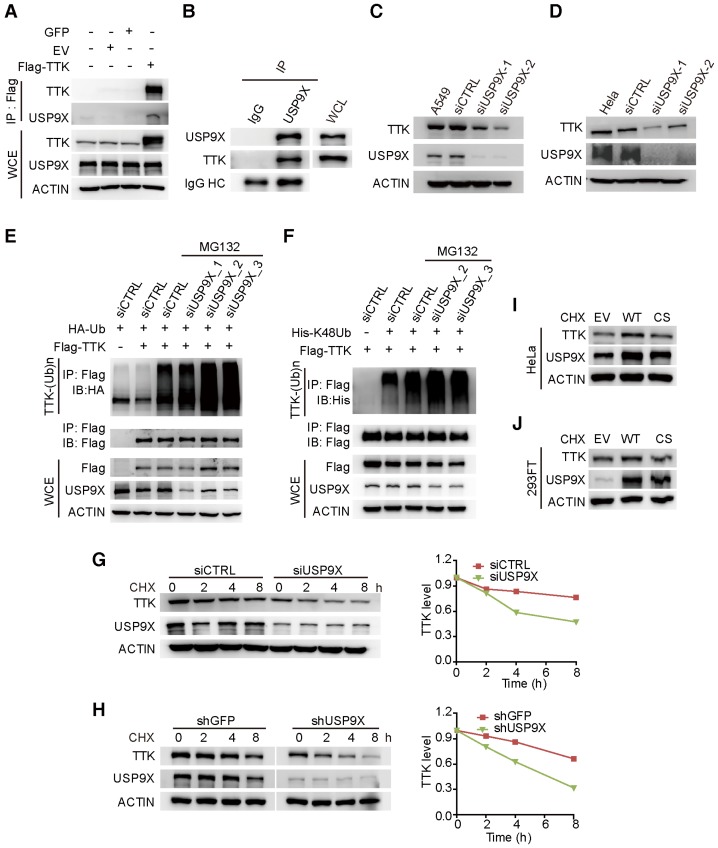
Figure 2.
USP9X is functionally linked to the stability of TTK. (A) GFP, empty vector control (EV), and flag-TTK were transfected into 293T cells. The cells were extracted for immunoprecipitation with the anti-flag agarose and the proteins were analyzed by immunoblotting. (B) Immunoprecipitated proteins with anti-USP9X antibodies or control IgG from 293T extracts were analyzed by western blotting. Endogenous TTK interacting with USP9X was detected using an anti-TTK antibody. (C) A549 cells were transfected with either two different siRNAs targeting USP9X or a control siRNA. After 72 h, cells were harvested and proteins were analyzed by immunoblotting. (D) The effect of USP9X depletion on TTK in HeLa cells as in (C). (E) Flag-TTK, HA-Ub, siRNA or siUSP9X were co-transfected into 293T cells. The cells were treated with or without MG132 as indicated. Then, cells were extracted for immunoprecipitation with anti-flag agarose and analyzed by western blotting. (F) Flag-TTK, ubiquitin K48-only plasmids, siRNA or siUSP9X were co-transfected into 293T cells. The indicated cells were treated with MG132 overnight. Then, cells were extracted for immunoprecipitation with anti-flag agarose and analyzed by western blotting. (G) A549 cells were transfected with either control or USP9X siRNAs. After 48 h, 200 μg/mL CHX was added and cells were harvested at the indicated times. Protein samples were analyzed by immunoblotting. Quantification of TTK levels relative to β-actin are presented. (H) Half-life analysis of TTK in constructed stable A549 cells. (I-J) HeLa (I) or 293FT (J) cells were transfected with plasmids expressing functional V5-USP9X (WT), catalytically dead C1566S (CS) USP9X or empty vector (EV). After 36 h, 200 μg/mL CHX was added and cells were harvested for immunoblotting.