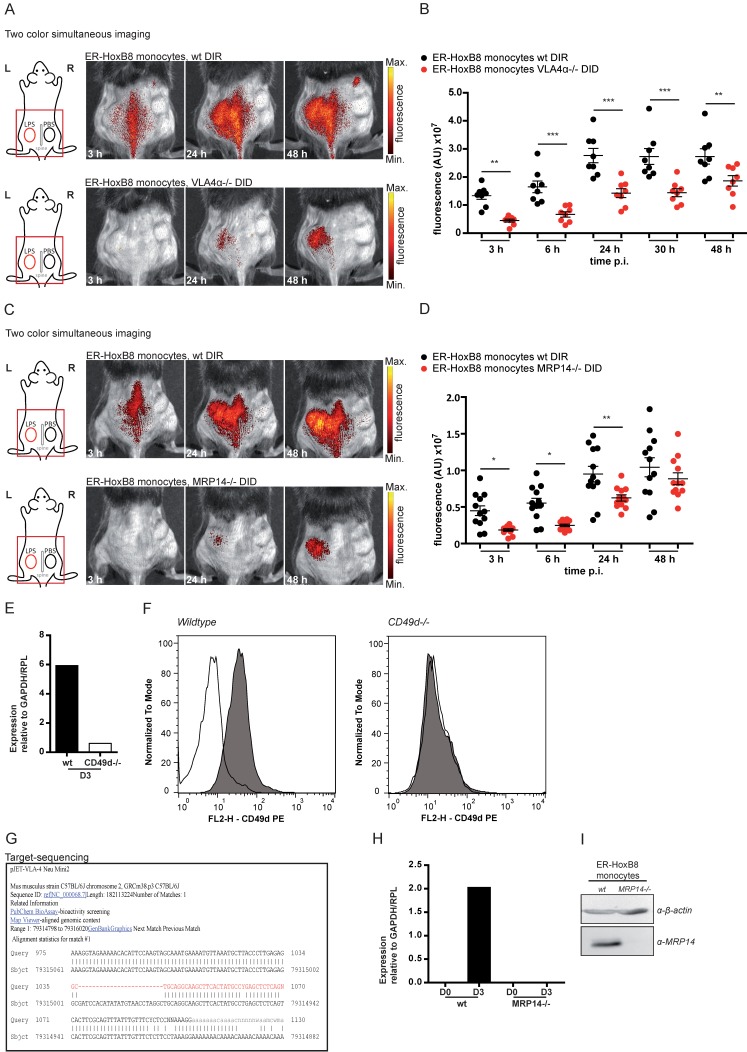
Figure 5.
In vivo optical imaging of VLA4α-/- and MRP14-/- monocyte migration in a CG model. ER-HoxB8 wildtype and VLA4α-/- monocytes (A-B) or wildtype and MRP14-/- monocytes (C-D) were differentially labeled with DIR or DID and injected in the same mouse. FRI images were taken 0 h, 3 h, 6 h, 24 h, 30 h and 48 h p.i. (A and C) Representative imaging series (3-48 h) of two color simultaneous imaging of LPS plug infiltration in a single mouse is shown for (A) wt monocytes (DIR, upper panel) and VLA4α-/- monocytes (DID, lower panel) and (C) wt monocytes (DIR, upper panel) and MRP14-/- monocytes (DID, lower panel). (B and D) Statistical analysis of simultaneous imaging of cell migration to LPS plug of (B) wt and VLA4α-/- monocytes corresponding to (A) (n=8 mice, 3 independent experiments) and (D) of wt and MRP14-/- monocytes corresponding to (C) (n=12 mice, 5 independent experiments). (E-G) Verification of VLA4α (CD49d) knockout in ER-HoxB8 VLA4α-/- monocytes. Representative results are shown. (E) qRT-PCR of wt and VLA4α-/- monocytes (day 3) for CD49d (VLA4α) mRNA. (F) Flow cytometry analysis of α-CD49d staining of wt and VLA4α-/- monocytes (day 3). CD49d expression was detected as FL2-H+. Open graphs show isotype control; grey graphs α-CD49d/VLA4α staining. (G) DNA-sequencing of the CRISPR/Cas9 target region of VLA4α-/- monocytes. (H-I) Verification of MRP14 knockout in ER-HoxB8 MRP14-/- monocytes. Representative results are shown. (H) qRT-PCR of wt and MRP14-/- monocytes (day 0 and day 3) for MRP14 mRNA. (I) WB analysis of wt and MRP14-/- monocytes (day 3) by α-MRP14 staining. Images orientation: L = left, R = right, fluorescence = fluorescence intensity (AU). Data are shown as dotplots with mean ± SEM, corrected to baseline and labeling efficiency. Statistical significance was calculated using 2-way ANOVA and Bonferroni post-tests comparing ER-HoxB8 wildtype and VLA4α-/- or MRP14-/- cells: *p < 0.05, **p < 0.01, ***p < 0.001.