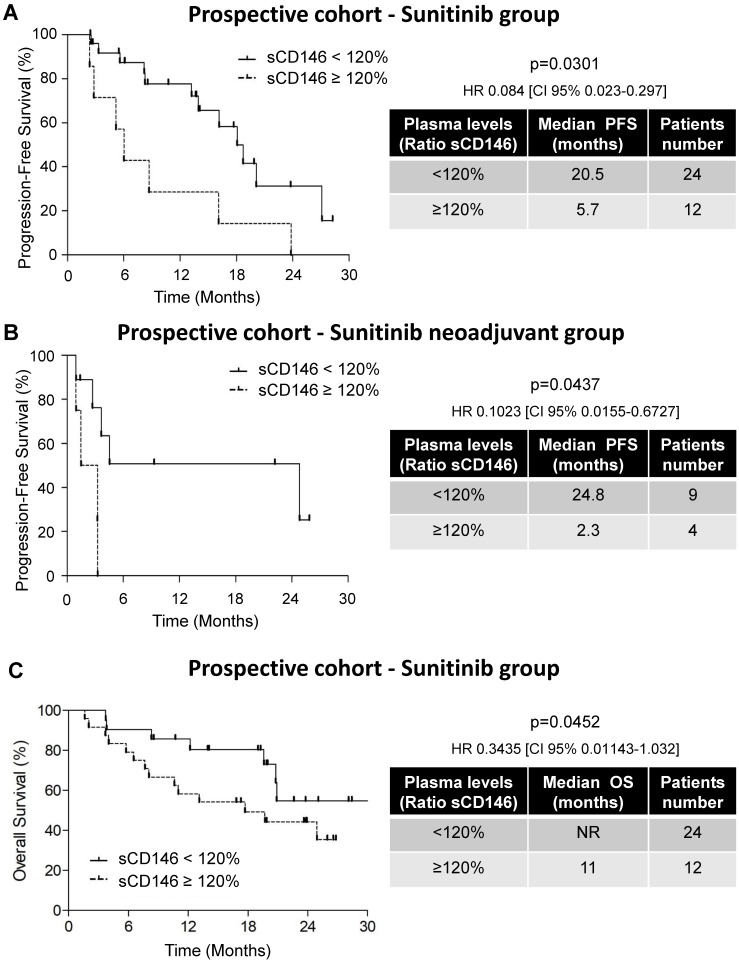
Figure 2.
Variations in the sCD146 plasmatic levels were indicative of PFS and OS for patients treated with sunitinib. (A and B) Kaplan-Meier analysis of PFS: the PFS was calculated from patient subgroups with a ratio of plasmatic levels for sCD146 obtained between diagnosis and after the first cycle, which was less or greater than a cut-off ratio of 120%, for SUVEGIL and TORAVA trial - sunitinib group (A) or for an independent cohort of patients treated in a neoadjuvant setting (B). (C) Kaplan-Meier analysis of OS: OS was calculated from patient subgroups with a ratio of plasmatic levels for sCD146 obtained between diagnosis and after the first cycle, which was less or greater than a cut-off ratio of 120%, for SUVEGIL and TORAVA trial - sunitinib group. Statistical significance (p value) and the time of PFS and OS are indicated (see Figure S4 and Table S2).