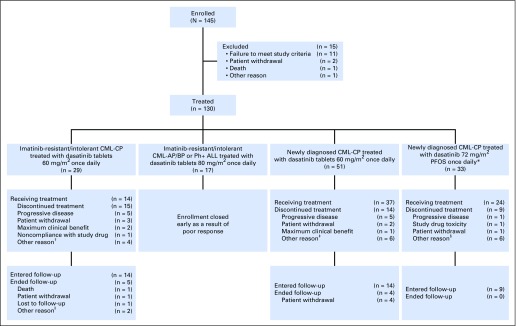
Fig 1.
CONSORT diagram of study CA180-226. (*)Patients had the option to switch to dasatinib tablets 60 mg/m2 once daily after 1 year of receiving PFOS. (†)Other includes bone marrow transplant, allogenic bone marrow graft, suboptimal response, and patient lost to follow-up. (‡)Other includes T315I mutation, loss of major molecular response, investigator decision, loss of complete cytogenetic response (n = 2), and hematopoietic stem cell transplant (n = 2). (§)Other includes hematopoietic stem cell transplant (n = 2), suboptimal response, second malignancy, and transition to adult hospital (n = 2). (ǁ)Other includes transfer to adult hospital and bone marrow transplant. CML-AP/BP, chronic myeloid leukemia in accelerated/blast phase; CML-CP, chronic myeloid leukemia in chronic phase; PFOS, powder for oral suspension; Ph+ ALL, Philadelphia chromosome-positive acute lymphoblastic leukemia.