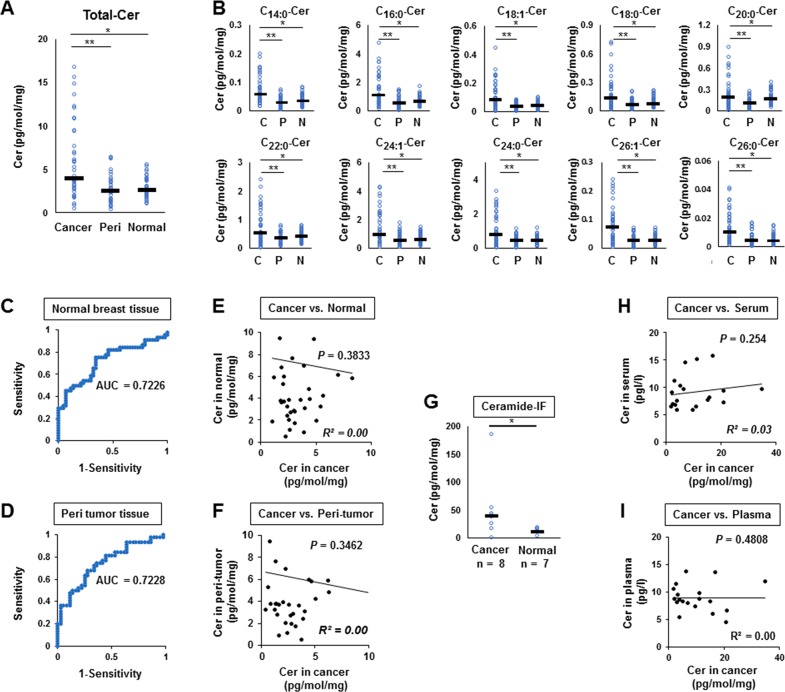
Figure 2. Ceramide levels in breast cancer tissue (n = 44), peri-tumor tissue (n = 36), and normal breast tissue (n = 44).
(A, B), Levels of total ceramide (Cer) (A) and each ceramide specie (C14:0, C16:0, C18:1, C18:0, C20:0, C22:0, C24:1, C24:0, C26:1 and C26:0) (B) in cancer tissue (Cancer or C), peri-tumor tissue (Peri or P) and normal tissue (Normal or N) were determined by mass spectrometry. Mean values are shown by the horizontal lines. *, P<0.05 for cancer vs. normal tissue; **, P<0.05 for cancer vs. peri-tumor tissue. (C, D), Receiver-operating characteristic (ROC) curves and the area under the ROC curve (AUC) were produced to assess the ability of our ceramide assays to distinguish cancer tissue from either normal breast tissue sample (C) or peri-tumor tissue (D). (E, F), Correlation between the ceramide level in breast cancer tissue and that in normal breast tissue (E) or peri-tumor (F) were compared in individual patient. The correlation between two variables is denoted by R2. (G) The ceramide levels in interstitial fluid (IF) of cancer tissue and normal breast tissue were determined. (H, I) Correlation between the ceramide level in breast cancer tissue and that in serum (H) or plasma (I) were compared. The correlation between two variables is denoted by R2.