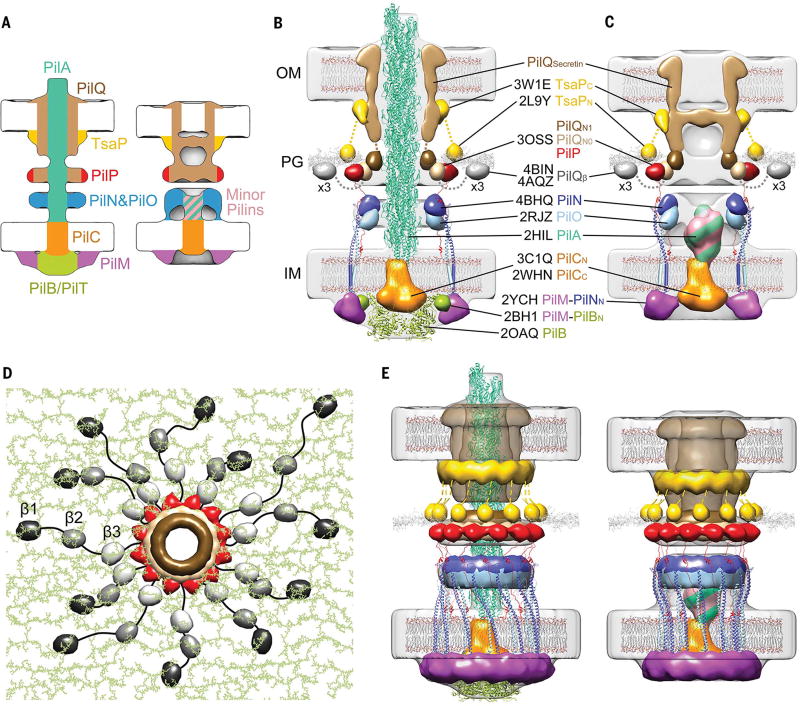
Fig.3. Architectural models of the T4PM.
(A) Summary schematics showing the component locations identified in the piliated and empty T4PM basal body structures. (B and C) Central slices of the architectural models of piliated and empty T4PM basal bodies, respectively, in which atomic models of T4PM components are placed in the in vivo envelopes according to the component maps in (A) and previously reported constraints and filtered to 3-nm resolution. (The process of how each component was placed is detailed in Movie 1 and the supplementary materials.) Models of each component are colored as in (A), with the transmembrane segments of PilN and PilO shown as cylinders; “x3” indicates three AMIN domains per PilQ monomer, only one of which is shown. Note that the empty T4PM basal body is shown with five PilA major pilin subunits in the short stem; however, the short stem likely also contains minor pilins. (D) Top view of the PilP HR domains and the PilQ N0 and N1 domains in the architectural model [colored as in (B) and (C)], with PG model (colored green) as background; 36 AMIN (β) domain models from 12 PilQ proteins are randomly placed on PG and connected by long flexible linkers (black) with lengths within 20 nm between β1 and β2 domains (70 residues), 12 nm between β2 and β3 domains (40 residues), and 12 nm between β3 and N0 domains (40 residues). (E) Overall architectural models of piliated (left) and empty (right) T4PM basal bodies. For clarity, the PilQ AMIN domains displayed in (D) are not shown.