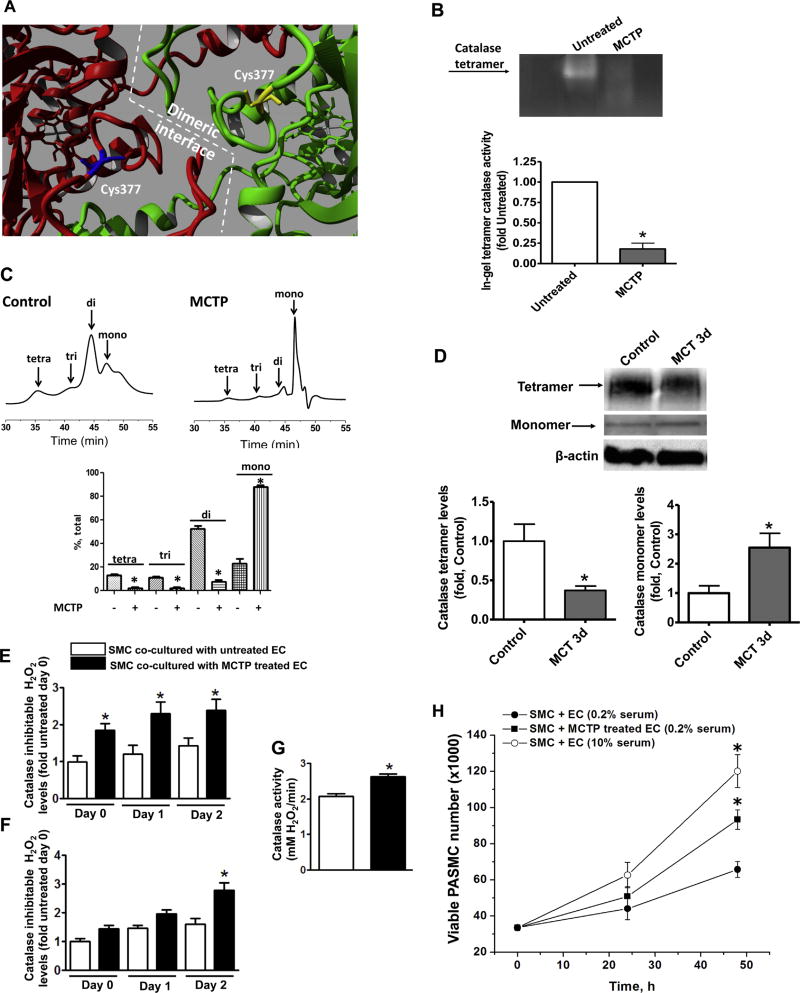
Fig. 2.
The monocrotaline pyrrole adduct disrupts the oligomeric structure of catalase. Analysis of the crystal structure of catalase (PBD ID 1QQW) identified C377 as being located at the dimeric interface (A). Semi-native zymography (in gel activity) demonstrates that the activity of recombinant catalase tetramer is significantly attenuated by MCTP (30 µM, 30 min) (B). Analytical gel filtration confirms the disruption of the catalase multimeric structure by MCTP (C). Semi-native gel electrophoresis of peripheral lung tissues of MCT-treated rats and the following western blot analysis identified a significant reduction in the tetrameric form of catalase and accumulation of catalase monomer (D). Pretreatment of PAEC with MCTP (200 µM, 30 min), followed by complete removal of MCTP, causes a significant increase in H2O2 in the media within 2 h and this persists for at least 48 h (E). H2O2 levels increase in the PASMC co-cultured with PAEC pretreated with MCTP after 48 h (F) despite a significant increase in catalase activity (G). The increase in H2O2 in the PASMC correlates with an increase in proliferation (MTS proliferation assay) (H). Results are expressed as mean±SEM; N=3–4. *P<0.05 vs. control or untreated group.