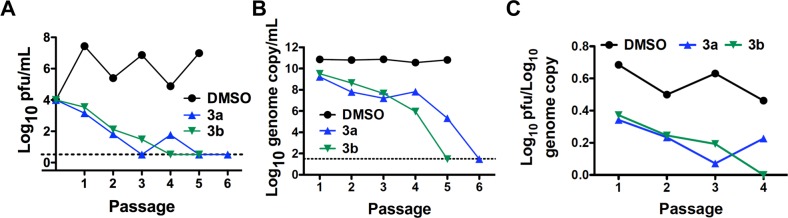
Fig 4. Anti-DENV effects during serial passage of virus in the presence of antiviral nucleobase and nucleosides.
Virus passaged in Huh-7 cells in the presence of 3a (200 μM, blue) and 3b (500 μM, green). Cells were initially inoculated at an m.o.i. of 0.01 and cultured in compound at the indicated concentration. Every 72 hours a fixed volume of supernatant was used to inoculate fresh cells maintained in compound. A. The titer of virus in the supernatant was obtained by serial dilution and counting plaques in single dilution containing 20–50 plaques when possible (see Methods). This experiment was repeated three times at these compound concentrations with similar results and a representative experiment is shown. The dashed line indicates the limit of detection of 3.3 pfu/mL (see Methods). B. Genome copy equivalents were monitored for the passages indicated using RT-qPCR. Mean values and standard deviations of duplicate measurements are shown. All points have standard deviations represented by error bars however some error bars may be too small to be seen. This experiment was performed twice with similar results. The dashed line indicates the limit of detection of 30 copies of NS5 gene (see Methods). C. Specific infectivity was calculated as log10 titer divided by log10 genome copy equivalents for each passage and treatment.