Fig. 2. Clusters of basic amino acid residues in CD28CD are required for phosphorylation by Lck.
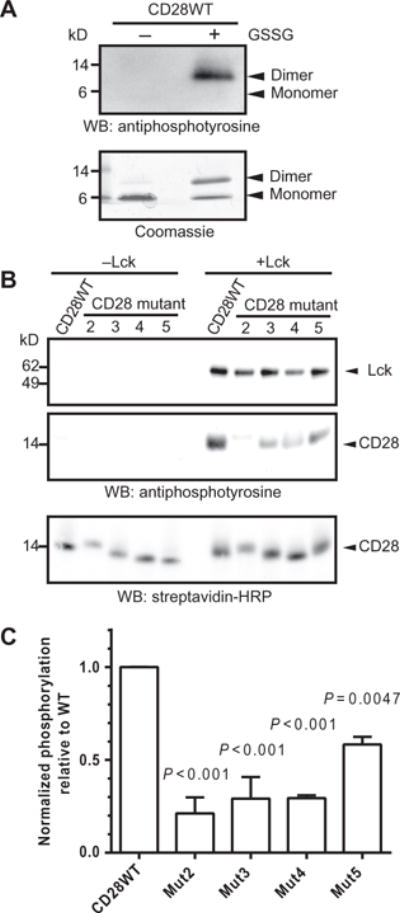
(A) Purified CD28WTCD was subjected to an in vitro phosphorylation assay with recombinant Lck in the presence or absence of the oxidizing agent GSSG. The reaction products were visualized by Western blotting (WB) analysis with antiphosphotyrosine antibody (top) and by Coomassie gel staining (bottom). Data are representative of two experiments. (B) Monobiotinylated, N-terminally linked dimers of the WT and indicated mutant CD28CD proteins were subjected to in vitro phosphorylation assay (right), or as a control, were incubated in the absence of Lck (left). Reaction products were visualized by Western blotting analysis with an antiphosphotyrosine antibody, which detects both phosphorylated Lck and phosphorylated CD28 as indicated by molecular weight standards, and with SA to show equivalent loading of CD28 proteins. (C) Densitometric analysis was performed for reactions containing Lck. The extent of phosphorylation was determined by dividing the intensity of the indicated CD28 protein bands on the antiphosphotyrosine Western blot by that of the SA loading control for each sample. The extent of phosphorylation of the mutant CD28 proteins was compared to the average of the CD28 WT samples. Data are means ± SD of two experiments. The mean normalized amount of phosphorylated CD28WT was compared to the mean of each other group for statistically significant differences by one-way ANOVA with Dunnett’s correction.
