Figure 8.
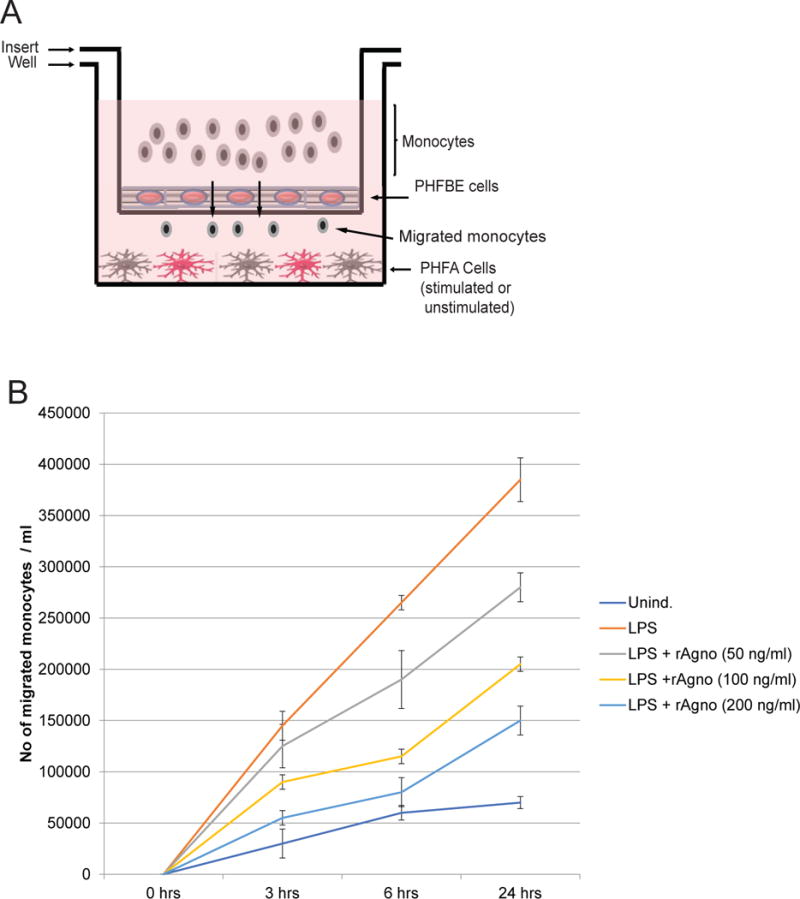
Agnoprotein decreases monocyte migration across a blood-brain barrier model. A. Schematic representation of the blood-brain barrier model used for the study. A corning 3.0 μm trans-well was inserted into a 6-well tissue culture dish. The 6-well tissue culture dish was seeded with PHFA that were stimulated with LPS or left unstimulated. The trans-well insert was seeded with primary human fetal brain endothelial (PHFBE) cells to form a confluent surface mimicking the blood-brain barrier. Primary human monocytes were seeded into the top chamber of the insert and allowed to migrate across the BBB-like membrane into the bottom portion of the system. Media from the bottom portion of the system was assessed for the presence of monocytes using cell-counting. B. PHFA cells were treated with recombinant agnoprotein at concentrations of 50, 100, and 200ng/mL. At 24 hours, cells were retreated with recombinant agnoprotein and simultaneously activated with LPS treatment. At 24 hours, LPS-containing media was removed and cells were retreated with agnoprotein in combination with the addition of one million human primary monocytes. Migrated cells in the bottom well were counted at 3, 6, and 24 hours after their addition into the top chamber. Experiment was completed in triplicate and standard deviations were calculated from the triplicates.
