Abstract
Although emerging neuropsychological evidence supports the involvement of temporal areas, and in particular the right superior temporal gyrus (STG), in allocentric neglect deficits, the role of STG in healthy spatial processing remains elusive. While several functional brain imaging studies have demonstrated involvement of the STG in tasks involving explicit stimulus-centered judgments, prior rTMS studies targeting the right STG did not find the expected neglect-like rightward bias in size judgments using the conventional landmark task. The objective of the current study was to investigate whether disruption of the right STG using inhibitory repetitive transcranial magnetic stimulation (rTMS) could impact stimulus-centered, allocentric spatial processing in healthy individuals. A lateralized version of the landmark task was developed to accentuate the dissociation between viewer-centered and stimulus-centered reference frames. We predicted that inhibiting activity in the right STG would decrease accuracy because of induced rightward bias centered on the line stimulus irrespective of its viewer-centered or egocentric locations.
Eleven healthy, right-handed adults underwent the lateralized landmark task. After viewing each stimulus, participants had to judge whether the line was bisected, or whether the left (left-long trials) or the right segment (right-long trials) of the line was longer. Participants repeated the task before (pre-rTMS) and after (post-rTMS) receiving 20 minutes of 1 Hz rTMS over the right STG, the right supramarginal gyrus (SMG), and the vertex (a control site) during three separate visits. Linear mixed models for binomial data were generated with either accuracy or judgment errors as dependent variables, to compare 1) performance across trial types (bisection, non-bisection), and 2) pre- vs. post-rTMS performance between the vertex and the STG and the vertex and the SMG.
Line eccentricity (z = 4.31, p < .0001) and line bisection (z = 5.49, p < .0001) were significant predictors of accuracy. In the models comparing the effects of rTMS, a significant two-way interaction with STG (z = −3.09, p = .002) revealed a decrease in accuracy of 9.5% and an increase in errors of the right-long type by 10.7% on bisection trials, in both left and right viewer-centered locations. No significant changes in leftward errors were found. These findings suggested an induced stimulus-centered rightward bias in our participants after STG stimulation. Notably, accuracy or errors were not influenced by SMG stimulation compared to vertex.
In line with our predictions, the findings provide compelling evidence for right STG’s involvement in healthy stimulus-centered spatial processing.
Keywords: Allocentric, egocentric, spatial processing, spatial neglect, right superior temporal gyrus, landmark task
1. INTRODUCTION
In the history of behavioral neurology and cognitive neuroscience, the study of spatial neglect has been crucial to our understanding of normal spatial cognition in healthy individuals. Spatial neglect, a frequent occurrence after stroke, is characterized as an inability to attend to, perceive, or plan motor responses toward stimuli presented on the side opposite to the injured cerebral hemisphere (Heilman & Valenstein, 1979; Mesulam, 1981). There are a number of symptom features in neglect that not only define behavioral phenotypes of the disorder (Barrett & Burkholder, 2006; Buxbaum et al., 2004) but also help unravel complex aspects of intact spatial processing. One of these features is the distinction between egocentric and allocentric spatial processing deficits. These clinically dissociable deficits provide support for the existence of multiple spatial reference systems, which when disrupted result in distinct spatial impairments.
Egocentric deficits result from disrupted processing with respect to reference frames centered on the self —the viewer— or one’s body parts (e.g. retina, head, trunk, shoulder). By contrast, allocentric deficits— often referred to as stimulus-centered deficits—are centered on the stimulus or object in view, and depend on the properties of the stimulus, including its orientation in space (Medina et al., 2009). Lesion studies suggest that reference-frame based spatial deficits arise from damage to distinct but related areas in the brain (Chen, Caulfield, Hartman, O'Rourke, & Toglia, 2016; Khurshid et al., 2012; Marsh & Hillis, 2008; Medina et al., 2009; Pouget & Driver, 2000). The right supramarginal gyrus (SMG; BA 40) and angular gyrus (BA 39) in the posterior parietal cortex (PPC) are often implicated in egocentric deficits (Hillis et al., 2005; Medina et al., 2009), whereas damage to the temporal areas such as the superior temporal gyrus (STG; BA 22), and the middle and inferior temporal gyri and their surrounding white matter (Grimsen, Hildebrandt, & Fahle, 2008; Hillis et al., 2005; Medina et al., 2009; Shah, Spaldo, Barrett, & Chen, 2013; Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010) are frequently associated with allocentric deficits.
While the evidence from neuropsychological studies is compelling, more direct evidence of distinct anatomical substrates of reference-frame based spatial processing in intact systems still remains elusive (Fink et al., 2003). One of the main reasons for this gap is that when studying brain-behavior relationships in lesion studies, it is difficult to differentiate brain areas that uniquely contribute to intact functions from those that have undergone functional reorganization in response to brain injury (Muggleton et al., 2006). In recent years, repetitive transcranial magnetic stimulation (rTMS) has proven to be a useful tool to address this dilemma, as it has the ability to induce focal and reversible changes in neural function. These changes are by definition acute and transient in nature, and therefore largely circumvent the issue of functional reorganization posed by typical lesion studies (Pascual-Leone, Walsh, & Rothwell, 2000).
A review of several prior rTMS studies (Ellison, Schindler, Pattison, & Milner, 2004; Oliveri & Vallar, 2009), studies using intraoperative stimulation (Gharabaghi, Fruhmann Berger, Tatagiba, & Karnath, 2006), and fMRI studies in healthy individuals paints a complex picture of the anatomical underpinnings of reference-frame based spatial processing. In particular, there is mixed evidence with respect to the role of right STG in allocentric processing. A small number of fMRI studies in healthy individuals link activation in the right STG (Neggers, Van der Lubbe, Ramsey, & Postma, 2006) with allocentric judgment task demands (Galati et al., 2000). However, a study by Ellison and colleagues (2004) demonstrated that inhibitory rTMS of the right PPC, but not the right STG, biased judgments in a landmark task (Ellison et al., 2004)—a task that is commonly used in the diagnosis of spatial neglect (Harvey, Milner, & Roberts, 1995). Oliveri and Vallar (2009) also demonstrated that disrupting activity in the right PPC (specifically SMG), but not the right STG, produced neglect-like rightward errors in a landmark task (Oliveri & Vallar, 2009). The landmark task is widely used to dissociate perceptual and premotor aspects of spatial neglect after stroke (Bisiach, Ricci, Lualdi, & Colombo, 1998; Harvey, Kramer-McCaffery, Dow, Murphy, & Gilchrist, 2002; Harvey et al., 1995) and in healthy individuals to assess intrinsic spatial bias (Jewell & McCourt, 2000; McCourt, 2001). We reasoned that this task in its conventional form does not appropriately impose allocentric processing demands, which may explain why prior rTMS studies did not find neglect-like behaviors after right STG stimulation. In the current study, we examined this relationship using an inhibitory rTMS paradigm in healthy individuals. Specifically, we interrogated the involvement of the right STG and right SMG using a task requiring allocentric judgments with the prediction that disrupting right STG, but not the right SMG, would selectively impact our participants’ allocentric processing abilities. To test our hypothesis, we modified the landmark task to increase its allocentric processing demand.
In conventional landmark tasks, participants are asked to judge whether pre-marked horizontal lines (a short vertical line intersecting a long horizontal line) are bisected, or whether the right- or left-segment of the lines are longer (or shorter) than the other. Thus the task probes perceptual judgments with no explicit motor response, which minimizes engagement of premotor processes. Importantly, the pre-marked lines are almost always displayed at the center of a computer screen that is aligned with the egocentric or viewer’s center. Because the center with respect to the viewer and the center of the stimuli (i.e., lines) are identical, it is difficult to discriminate between the egocentric and allocentric frames, since the left side of the line and the left with respect to the viewer are the same. To address this issue, presentation of pre-marked lines was lateralized in the present study. The lines were displayed either to the perceived left or right of the viewer (McCourt, Garlinghouse, & Slater, 2000). Because of the lateralized presentation, the viewer-centered (egocentric) and stimulus-centered (allocentric) coordinates were no longer identical. A pre-marked line displayed to the right of the viewer would have distinct left and right coordinates with respect to the stimulus; additionally, the left of the line would be in viewer-centered right space thus disambiguating between egocentric and allocentric requirement of spatial processing. We refer to this revised version of the landmark task as the “lateralized landmark task”.
We posited that the lateralized display in two egocentric positions (also referred to as viewer-centered), and allocentric judgment conditions (also referred to as stimulus-centered) would allow us to better discriminate and manipulate the stimulus-centered component of task performance using rTMS. In three separate stimulation sessions, we used low-frequency (1 Hz) rTMS to stimulate the right STG, right SMG, and a control site (vertex) before and immediately after the participants completed the lateralized landmark task. Based on the literature-suggested roles of right STG and right SMG in spatial reference frames, we had three predictions. 1) Inhibiting the right STG would increase judgment errors biased toward the contralateral side with respect to the line (stimulus) but not the viewer. Specifically, for bisected lines, the prediction is that allocentric neglect-like behavior would manifest as an increased proportion of errors that can be attributed to increased rightward bias regardless of the viewer-centered position of the line. 2) rTMS of the right SMG, on the other hand, would produce neglect-like rightward bias but only on the left viewer-centered side, as reported in several prior studies in healthy individuals (Oliveri & Vallar, 2009). 3) No change in behavior was expected after vertex stimulation. We used vertex stimulation as an active control to compare judgment errors after STG and SMG stimulation.
2. MATERIALS AND METHODS
2.1 Participants
Eleven right-handed individuals (8 females; mean age = 24.9 ± 8.02 years) with no history of neurological or psychiatric disorders participated in this study. None of the participants had any contraindications to receiving TMS. Only those participants for whom we had access to T1-weighted MRI scans from prior research studies were included. This study was approved by the local Institutional Review Board. All participants provided informed consent before any study procedures began.
2.2 Procedures
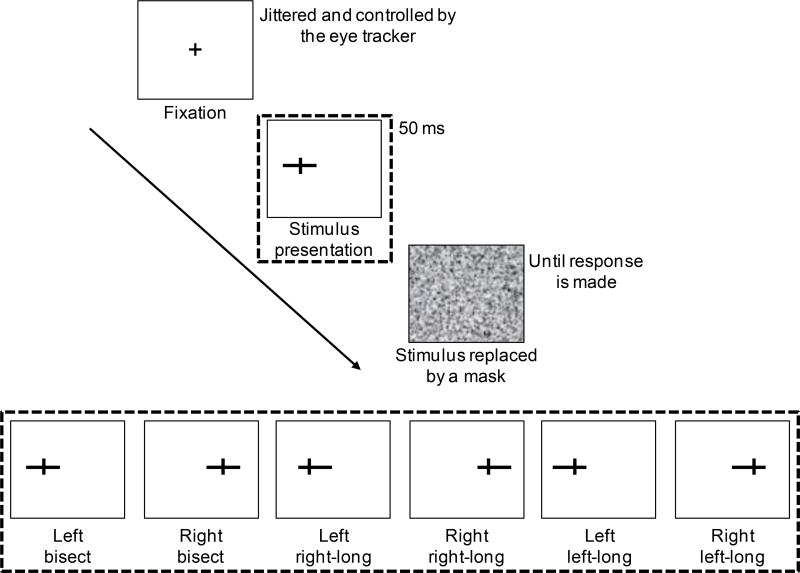
All participants visited the lab three times during the course of this study. During each visit, participants underwent the following five steps (Figure 1). 1) To introduce participants to the task and ensure high performance before brain stimulation, we presented participants with a practice version (48 trials) of the lateralized landmark task (section 2.4). At the end of the practice run, participants received feedback (accuracy) on their performance. To provide additional practice, participants underwent one additional practice run if they did not achieve 80% accuracy on the first run. 2) All participants then completed the full version of the task, which we refer to as the pre-rTMS block. 3) After the pre-rTMS block, resting motor threshold (rMT) was determined (section 2.3). 4) RTMS was administered for 20 minutes (section 2.3) at one of the three brain sites: vertex, right STG, or right SMG. 5) Immediately after the stimulation ended, participants repeated the full version of the task, which we refer to as the post-rTMS block. The same five steps were repeated during all three visits but with a different site of stimulation.
Figure 1.
Study procedures during each visit. rTMS = repetitive transcranial magnetic stimulation; rMT = resting motor threshold; MEP = Motor Evoked Potential
2.3 rTMS paradigm
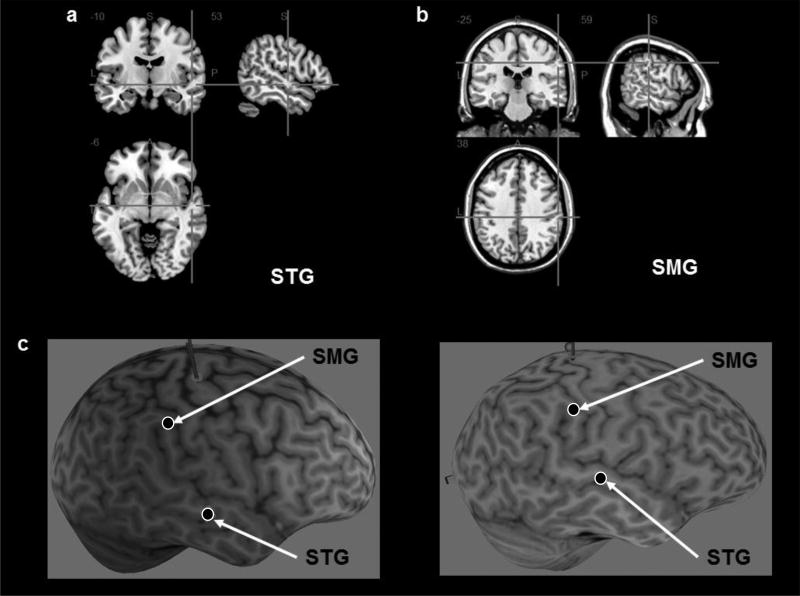
Stimulation was administered using the Magstim Super Rapid2plus1 transcranial magnetic stimulator connected to a 70-mm diameter figure-of-eight, air-cooled coil (Magstim, Whitland, UK). Participants’ T1-weighted MRI scans were uploaded to the Brainsight® Neuronavigation system (Rogue Research, Montreal) and were used to identify the stimulation sites. The right STG was localized using the MNI coordinates (48, −20, −8) from Neggers et al. (2006) study, the right SMG was localized using coordinates (63, −37, 49) identified in Oliveri and Vallar (2009). Subsequently, a neurologist (author RH) verified the locations and ensured consistency in mapping the stimulation sites across participants over the course of the study (Figure 2).
Figure 2.
The right superior temporal gyrus (a; STG) and the right supramarginal gyrus (b; SMG) were localized for each participant; illustrations of STG and SMG site selections in 2 representative participants (c).
An rMT was defined as the minimum stimulation intensity at which minimum of 5 out of 10 motor-evoked potentials (MEP) were obtained at or less than 50µV peak-to-peak amplitude; the MEPs were acquired from the first dorsal interosseous in all participants. RMTs were recorded during each visit to account for day-to-day variability in cortical excitability. Stimulation was then delivered at an intensity of 110% of rMT for that visit at a frequency of 1 Hz for 20 minutes, for a total of 1200 pulses. The order of the site of stimulation was pseudo-randomized across participants. A wait period of 24 hours was mandated between visits to avoid any carryover effects.
2.4 Lateralized Landmark Task
In the modified version of the landmark task, 96 pre-marked horizontal lines were presented either on the left (48 trials) or on the right (48 trials) side of a computer screen (17" TFT display; screen size: 13" × 11"; Figure 3). A vertical mark (width: 0.1cm; height: 0.4cm) on the horizontal lines either bisected the lines (bisection condition; 32 trials), or transected the lines so that the right segment was longer than the left (right-side longer or R-long condition; 32 trials), or the left was longer than the right (left-side longer or L-long condition; 32 trials); only one type of pre-marked line was displayed at a time on the screen. There were an equal number of trials in the left and the right view-centered hemifields (48 trials each side). Originally the experiment was designed with half the number of total trials, which were doubled after the first participant had completed all study procedures and the second participant had completed their first visit. The first participant received 16 trials per line type and the second participant received 16 trials per line type for their first visit (SMG rTMS) and for all other visits received the standard 32 trials. Note that excluding these two participants from analyses described in the following sections did not affect the final results (sections 3.1 and 3.2; supplementary Tables 1–3). The vertical mark appeared at the same distance from the center of the screen on all trials (4.3cm), while the horizontal line either moved towards or away from the center of the screen to bisect or elongate one side of the line. To avoid participants from adopting a strategy that was based on the total line size, horizontal lines varied in length (either 5.3cm or 5.65cm, all horizontal lines 0.1cm wide). When the line was not bisected, the vertical mark transected the line at 30% point of the total line length, either to the right or the left of the line. All stimuli were displayed on the screen equipped with an eye-tracking device (Tobii T120; 120Hz) that was calibrated to each participant before each session. Participants were seated in front of the monitor at a distance of 40cm, measured from eye level to the center of the screen. At this distance, the eccentricity (visual angle with respect to the fixation or the veridical center of the screen) of the vertical marker was constant at 6.18°. The range of eccentricities of the line endpoints distal to the center of the screen was 8.95°– 11.03° in both hemifields; the range of eccentricities for the line endpoints proximal to the center of the screen was 1.15°– 3.29°.
Figure 3.
The lateralized landmark task. Fixation at the vertical center of the screen was ensured by an eye-tracker. The cross was replaced by one of the line stimuli (shown in the bottom insert), which was displayed for 50 milliseconds. The stimulus was replaced by a mask until the participant responded using a keyboard. The lines were displayed either on the right side or the left side of the fixation. Participants were instructed to judge whether the lines were bisected, right segment longer than the left (right-long) or left segment longer than the right (left-long).
Each trial began with a fixation cross (+) which remained on the screen until the eye-tracker detected fixation at the center of the screen. The cross was then immediately replaced by one of the line types. The line was displayed for 50 milliseconds (ms) and was replaced by a white noise mask that covered the entire screen until the participants made a response (Figure 2). Participants used a keyboard to respond with their right hand whether the lines were bisected (press “9”), right-long (press “0”) or left-long (press “8”). They were instructed to respond to the line stimulus as quickly as possible. Reaction time per trial was recorded as the time it took the participants to respond after the stimulus presentation. The order of presentation of each stimulus type (bisection, L-long or R-long, presented in left or right viewer-centered hemifields) was randomized.
2.5 Statistical Analysis
Given that our data was binomial (binary coding of accuracy and errors per trial), we analyzed our data using binomial linear mixed models using R 3.4.2 (R Core Team, 2017), specifically its glmer function in the lmer4 and lmerTest packages, with family set as binomial (Bates, Maechler, Bolker, & Walker, 2015; Kuznetsova, Brockhoff, & Christensen, 2017). Linear mixed models take into account both fixed effects (i.e. effects related to the independent variables) and random effects (i.e. effects related to the individual subjects or items selected from a population, but not related to the independent variables) in the same model. Each model included by-subject random intercepts (taking into account the average performance of each participant) and slopes (controlling for the effect of a specific dependent variable on each participant), with separate slopes defined by the fixed effects in each model. Fixed effects and interactions were selected based on our a priori hypotheses of interest. To compare different models, we used the anova function in the stats package in R and performed a Chi-square test to determine if adding a fixed effect/interaction improved fit. Independent variables were contrast coded and mean centered.
3. RESULTS
The mean rMT in 11 participants was 60.6 % (± 6.0) of the maximum stimulator output, and the mean stimulation intensity at 110% of rMT was 66.8 (± 7.0) %. The overall reaction time across all pre-rTMS blocks was 770.0 (± 433.9) milliseconds (ms) and across post-rTMS blocks was 710.8 (± 388.0) ms. Because the lateralized landmark task is a perceptual task with limited motor demands (Cicek, Deouell, & Knight, 2009), as mentioned earlier we expected rTMS-induced disruptions to affect target detection and in turn the accuracy/errors. However, we did not have predictions regarding specific changes in latencies after rTMS at different sites. Therefore, in the following sections we have focused on changes in accuracy and errors, reflective of spatial shifts in perceptual judgments, rather than latencies.
3.1 Assessment of accuracy across different trial-types
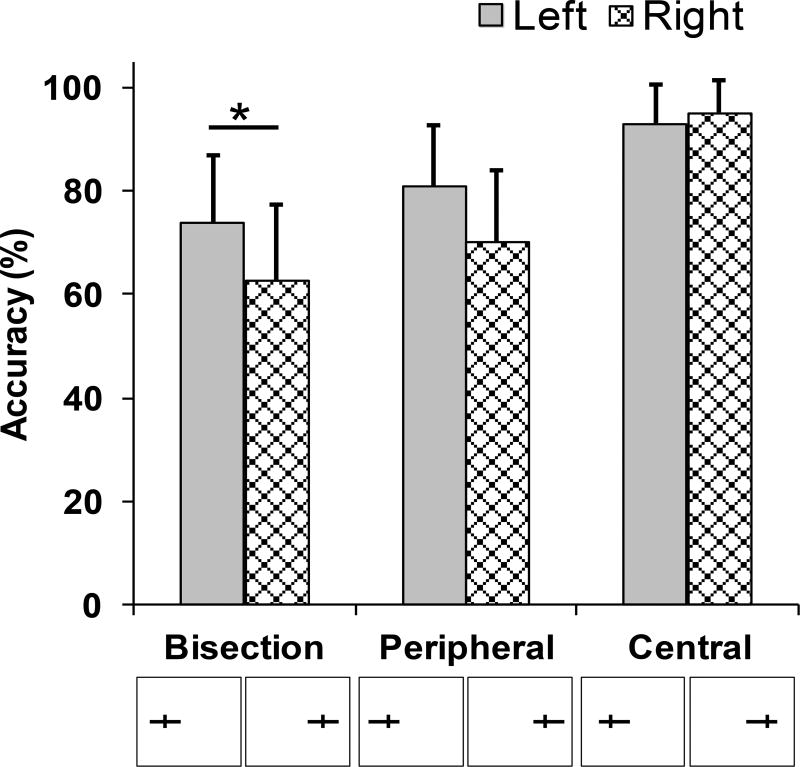
We first evaluated the differences in overall accuracy of our participants across the bisection and the non-bisection trial-types. As shown in Figure 4, participants were most accurate (94.1%) when the horizontal line was closer to fixation (i.e., central trials) versus farther from fixation (75.7% accuracy; peripheral trials), suggesting that line position relative to the tick mark influenced performance. However, the poorest performance (68.2% accuracy) was in the bisection trials suggesting that separate mechanisms were involved on these trials. Additionally, the accuracy did not differ across the pre-rTMS blocks in any of the trial-types (all p>0.05; accuracy in pre-rTMS blocks for vertex, SMG, STG, respectively in % are as follows: central trials: 94.9, 95.0, 94.3; peripheral trials: 78.9, 75.3, 74.4; bisection trials: 67.5, 67.2, 69.9), ruling out the possibility of practice effects.
Figure 4.
The accuracy on the lateralized landmark task. Participants were least accurate on the bisection trials [68.2%], while they were most accurate when the longer line segments appeared towards the center [94.1%]; the mean accuracies and standard errors are shown. Asterisk (*) indicates statistical significance at p<0.05.
To examine the differential effects of line eccentricity and line bisection further, we ran a linear mixed model with accuracy as the dependent variable and line eccentricity and line bisection as fixed effects. We found that both line eccentricity (z = 4.31, p < .0001) and line bisection (z = 5.49, p < .0001) were significant predictors of accuracy. Furthermore, the model with line bisection included was significantly more predictive than the model without line bisection (chi2 = 370.3, p < .0001). Based on this, we separately analyzed bisection trials and non-bisection trials (i.e., peripheral and central) to examine how rTMS influenced performance.
3.2 Pre- vs post-rTMS comparisons of accuracy and error-types
Next, the after effects of rTMS on accuracy were examined with the hypothesis that accuracy would be reduced after rTMS of the right STG irrespective of the viewer-centered position of the line, whereas rTMS of the right SMG would reduce accuracy only on the left viewer-centered side, both compared to the vertex rTMS; these hypotheses were initially tested on the bisection trials (section 3.2.1). We also evaluated the number and type of errors to ascertain the direction of the spatial bias that our participants exerted in response to rTMS.
3.2.1 Bisection trials
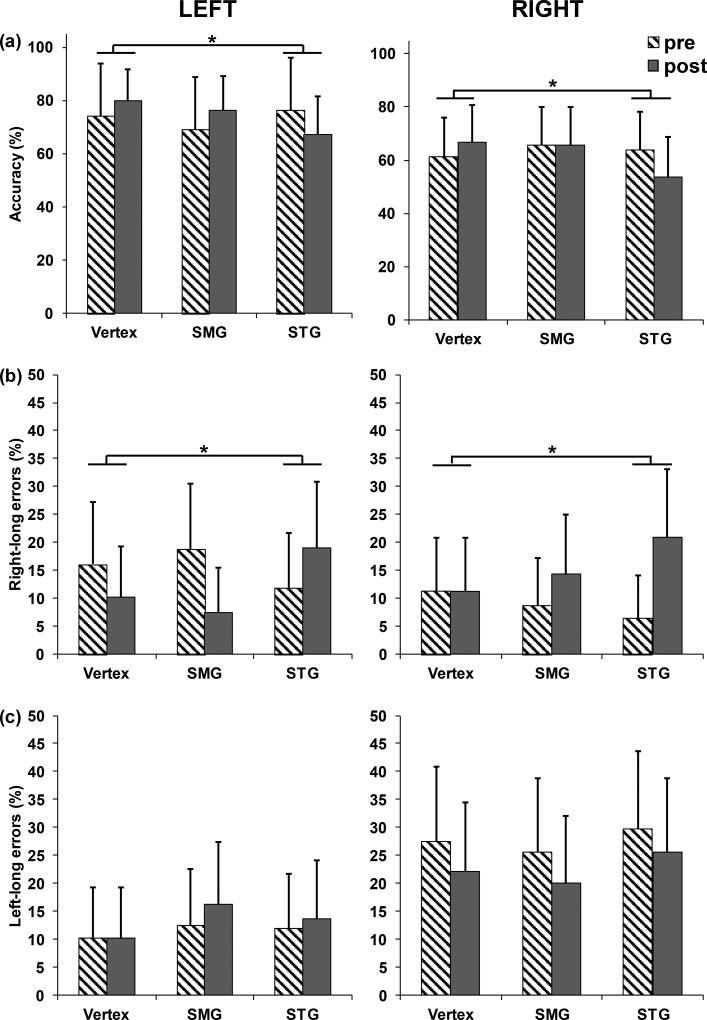
On bisection trials, we ran a linear mixed model with accuracy as the dependent variable and rTMS site (comparing vertex to SMG or STG), time (pre-rTMS, post-rTMS), egocentric line position (left or right of fixation) as fixed effects, with main effects and interactions for all factors. We found two significant effects. First, there was a main effect of egocentric line location (z = −2.21, p = .027), as participants were more accurate when the line was left of fixation (73.7%) versus right of fixation (62.7%). Importantly, there was a significant STG rTMS by time interaction (z = −3.09, p = .002; Table 1). Participants who received rTMS to vertex improved by 5.7% after stimulation (67.5% to 73.2%), whereas those who received STG rTMS showed a 9.5% decrease in performance (69.9% to 60.4%; Figure 5a; see supplementary Figure 1a for participant-wise changes) for lines presented in left and right of fixation. No other main effects or interactions were significant. This model provided evidence that STG rTMS decreased accuracy, but was not informative as to whether rTMS introduced a specific leftward or rightward bias that caused accuracy to reduce.
Table 1.
Summary of the fixed and random effects in the linear mixed model comparing accuracies. rTMS site has 3 levels (vertex, SMG, STG), viewer-centered or egocentric location (ego) of the line has 2 levels (left, right), time of stimulation has 2 levels (pre-rTMS, post-rTMS).
| Dependent variable: accuracy | ||||
|---|---|---|---|---|
|
| ||||
| Fixed Effects | Coefficient (b) | SE | z | Pr(>|z|) |
| SMG | −0.050 | 0.200 | −0.247 | 0.805 |
| STG | −0.285 | 0.256 | −1.111 | 0.267 |
| time | 0.215 | 0.222 | 0.971 | 0.331 |
| ego | −0.644 | 0.292 | −2.208 | *0.027 |
| SMG × time | −0.112 | 0.264 | −0.424 | 0.671 |
| STG × time | −0.799 | 0.259 | −3.09 | *0.002 |
| SMG × ego | 0.299 | 0.266 | 1.122 | 0.262 |
| STG × ego | 0.032 | 0.260 | 0.122 | 0.903 |
| time × ego | −0.033 | 0.371 | −0.089 | 0.929 |
| SMG × time × ego | −0.381 | 0.528 | −0.722 | 0.470 |
| STG × time × ego | 0.066 | 0.514 | 0.128 | 0.898 |
| Random Effects | Variance | |||
| (Intercept) | 0.128 | |||
| SMG | 0.210 | |||
| STG | 0.517 | |||
| (Intercept) | 0.432 | |||
| time | 0.140 | |||
| (Intercept) | 0.086 | |||
| ego | 0.531 | |||
| Residual | 0.014 | |||
See text for details. × signifies an interaction;
indicates statistical significance at p<0.05;
SE = standard error.
Figure 5.
(a) The STG rTMS by time interaction was significant in the model comparing accuracy in the bisection trials. Accuracy decreased by 9.5% after STG rTMS compared to vertex rTMS in both the left and right viewer-centered locations. (b) The decrease in accuracy was driven by an increase in errors of the right-long type by 10.7%, after STG rTMS as suggested by significant STG rTMS by time interaction in the model comparing the right-long errors. (c) No change in left-long errors was found after STG or SMG rTMS compared to the vertex. Error bars indicate standard errors. The left panel displays performance for lines presented left of the fixation (viewer-centered left), and the right panel for lines presented right of the fixation (viewer-centered right).
Therefore, we next evaluated the number and types of errors, i.e. right-long or left-long errors, on bisection trials. We ran two separate linear mixed models, one per each error-type. A significant STG rTMS by time interaction (z = 3.88, p < .001) was found in the model comparing the right-long errors (Table 2). Participants made 10.7% more right-long errors after STG rTMS (9.2% to 19.9%) whereas proportion of errors decreased by 3.0% after vertex rTMS (Figure 5b). No significant effects or interactions were significant in the model comparing the left-long errors (Table 3; Figure 5c). Refer to Supplementary Figure 1b and 1c for participant-wise changes in errors.
Table 2.
Summary of the fixed and random effects in the linear mixed model comparing right-long errors. rTMS site has 3 levels (vertex, SMG, STG), egocentric location (ego) of the line has 2 levels (left, right), time of stimulation has 2 levels (pre-rTMS, post-rTMS).
| Dependent variable: right-long errors | ||||
|---|---|---|---|---|
|
| ||||
| Fixed Effects | Coefficient (b) | SE | z | Pr(>|z|) |
| (Intercept) | −2.576 | 0.339 | −7.602 | <0.001 |
| SMG | 0.011 | 0.247 | 0.045 | 0.964 |
| STG | 0.159 | 0.409 | 0.389 | 0.698 |
| time | −0.186 | 0.273 | −0.683 | 0.494 |
| ego | 0.178 | 0.647 | 0.276 | 0.783 |
| SMG × time | 0.018 | 0.369 | 0.049 | 0.961 |
| STG × time | 1.410 | 0.363 | 3.879 | *<0.001 |
| SMG × ego | −0.057 | 0.379 | −0.149 | 0.881 |
| STG × ego | −0.454 | 0.414 | −1.096 | 0.273 |
| time × ego | 0.656 | 0.511 | 1.285 | 0.199 |
| SMG × time × ego | 1.214 | 0.737 | 1.646 | 0.100 |
| STG × time × ego | 0.150 | 0.722 | 0.208 | 0.835 |
| Random Effects | Variance | |||
| (Intercept) | 0.290 | |||
| SMG | 0.252 | |||
| STG | 1.365 | |||
| (Intercept) | 0.646 | |||
| time | 0.064 | |||
| (Intercept) | 0.027 | |||
| ego | 3.523 | |||
See text for details. × signifies an interaction;
indicates statistical significance at p<0.05;
SE = standard error.
Table 3.
Summary of the fixed and random effects in the linear mixed model comparing left-long errors. rTMS site has 3 levels (vertex, SMG, STG), egocentric location (ego) of the line has 2 levels (left, right), time of stimulation has 2 levels (pre- rTMS, post-rTMS).
| Dependent variable: left-long errors | ||||
|---|---|---|---|---|
|
| ||||
| Fixed Effects | Coefficient (b) | SE | z | Pr(>|z|) |
| (Intercept) | −2.019 | 0.309 | −6.544 | 0.000 |
| SMG | −0.018 | 0.252 | −0.073 | 0.942 |
| STG | 0.175 | 0.230 | 0.763 | 0.445 |
| time | −0.137 | 0.266 | −0.515 | 0.607 |
| ego | 0.728 | 0.530 | 1.373 | 0.170 |
| SMG × time | 0.172 | 0.332 | 0.517 | 0.605 |
| STG × time | 0.150 | 0.324 | 0.464 | 0.642 |
| SMG × ego | −0.347 | 0.343 | −1.011 | 0.312 |
| STG × ego | 0.040 | 0.335 | 0.118 | 0.906 |
| time × ego | −0.406 | 0.472 | −0.859 | 0.390 |
| SMG × time × ego | −0.415 | 0.659 | −0.630 | 0.529 |
| STG × time × ego | −0.089 | 0.645 | −0.138 | 0.890 |
| Random Effects | Variance | |||
| (Intercept) | 0.440 | |||
| SMG | 0.280 | |||
| STG | 0.215 | |||
| (Intercept) | 0.019 | |||
| time | 0.107 | |||
| (Intercept) | 0.371 | |||
| ego | 2.301 | |||
See text for details. × signifies an interaction;
indicates statistical significance at p<0.05;
SE = standard error.
3.2.2 Non-bisection trials
Finally, we ran a separate model for non-bisection trials, with rTMS site, time, line eccentricity, and egocentric line position as fixed effects, with interactions for all factors (Table 4). Consistent with results reported earlier, we found a significant line eccentricity effect (z = 4.60, p < .0001). Furthermore, there were two other significant effects—SMG by non-bisection trial types (z = −2.82, p = 0.005) and SMG by time by non-bisection trial types (z = −2.79, p = 0.005). However, owing to high collinearity among the independent variables, we are not confident in these effects and therefore have chosen to err on the side of caution and deem these effects uninterpretable.
Table 4.
Summary of the fixed and random effects in the linear mixed model comparing accuracy for the non-bisection trial types with central and peripheral eccentricities. rTMS site has 3 levels (vertex, SMG, STG), egocentric location (ego) of the line has 2 levels (left, right), time of stimulation has 2 levels (pre-rTMS, post-rTMS).
| Dependent variable: accuracy | ||||
|---|---|---|---|---|
|
| ||||
| Fixed Effects | Coefficient (b) |
SE | z | Pr(>|z|) |
| (Intercept) | 2.478 | 0.223 | 11.096 | <0.001 |
| SMG | −0.130 | 0.338 | −0.386 | 0.700 |
| STG | −0.029 | 0.208 | −0.141 | 0.888 |
| time | −0.108 | 0.236 | −0.457 | 0.648 |
| eccentricity | 2.287 | 0.497 | 4.600 | *<0.001 |
| egocentric line location (ego) | −0.309 | 0.393 | −0.786 | 0.432 |
| SMG × time | −0.085 | 0.290 | −0.292 | 0.771 |
| STG × time | 0.255 | 0.289 | 0.880 | 0.379 |
| SMG × eccentricity | −0.882 | 0.312 | −2.822 | *0.005 |
| STG × eccentricity | −0.401 | 0.301 | −1.329 | 0.184 |
| time × eccentricity | 0.751 | 0.427 | 1.756 | 0.079 |
| SMG × ego | −0.257 | 0.300 | −0.856 | 0.392 |
| STG × ego | −0.148 | 0.294 | −0.504 | 0.614 |
| time × ego | 0.248 | 0.422 | 0.588 | 0.556 |
| non-bisect × ego | 0.386 | 0.435 | 0.888 | 0.375 |
| SMG × time × eccentricity | −1.606 | 0.575 | −2.793 | *0.005a |
| STG × time × eccentricity | −0.854 | 0.577 | −1.479 | 0.139 |
| SMG × time × ego | −0.458 | 0.574 | −0.797 | 0.425 |
| STG × time × ego | −0.402 | 0.576 | −0.698 | 0.485 |
| SMG × eccentricity × ego | 0.723 | 0.579 | 1.249 | 0.212 |
| STG × eccentricity × ego | 0.278 | 0.580 | 0.480 | 0.631 |
| time × eccentricity × ego | 0.849 | 0.841 | 1.010 | 0.313 |
| SMG × time × eccentricity × ego | −0.303 | 1.148 | −0.264 | 0.792 |
| STG × time × eccentricity × ego | −1.615 | 1.152 | −1.402 | 0.161 |
| Random Effects | Variance | |||
| (Intercept) | 0.171 | |||
| SMG | 0.972 | |||
| STG | 0.206 | |||
| (Intercept) | 0.086 | |||
| time | 0.100 | |||
| (Intercept) | 0.001 | |||
| eccentricity | 2.018 | |||
| (Intercept) | 0.104 | |||
| ego | 1.128 | |||
See text for details. × signifies an interaction;
indicates statistical significance at p<0.05;
SE = standard error.
After SMG stimulation, accuracy decreased for central trials by 4.7% and increased for peripheral trials by 2.5%, compared to 0.3% increase for central and 8.3% decrease for peripheral after vertex stimulation. These results may indicate differential after effects of SMG stimulation on non-bisection trial types compared to vertex stimulation. We interpret these results with caution because of high collinearity among the independent variables in this model.
4. DISCUSSION
In this study, we provide compelling evidence for the right STG’s involvement in allocentric, stimulus-centered spatial processing in healthy individuals. Task accuracy was reduced after the right STG rTMS on trials where lines were bisected. Notably, reductions in accuracy were independent of the line’s position with respect to the viewer or the participant. Furthermore, there were significantly more right-long errors after right STG rTMS compared to a control site. To produce such errors, participants likely neglected the left side of the line itself, which falls on the side contralateral to right STG stimulation. Importantly, they under-estimated the left side of the line irrespective of their viewer-centered presentation—both when the lines were presented to participants’ left and right. This behavior was not found after right SMG or vertex stimulation. Allocentric neglect-like behavior was therefore induced selectively after right STG stimulation as evidenced from increased stimulus-centered rightward bias. These findings are consistent with lesion studies (Grimsen, Hildebrandt, & Fahle, 2008; Hillis et al., 2005; Medina et al., 2009; Shah, Spaldo, Barrett, & Chen, 2013; Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010) that found a selective role of right STG in allocentric neglect deficits, and also fMRI studies in healthy individuals that linked activation in the right STG (Neggers et al., 2006) to allocentric judgment task demands (Galati et al., 2000).
While our data supported the hypothesis regarding right STG’s role in allocentric processing, we did not find evidence supporting the right SMG’s role in egocentric processing. There are several prior rTMS studies in healthy individuals that reported neglect-like rightward bias after disrupting activity in the right PPC (Ellison et al., 2004; Muggleton et al., 2006) and specifically after disrupting the right SMG (Oliveri & Vallar, 2009). Prior evidence from fMRI studies also suggests a crucial role of the right parietal cortex in egocentric spatial processing, which in turn facilitates the mechanisms for action planning (Anderson, 1996; Driver, Baylis, Goodrich, & Rafal, 1994; Goodale, Westwood, & Milner, 2004; Kinsbourne, 1987). We therefore hypothesized that right SMG would increase the errors in the viewer-centered left but not on the right side. However, we did not find such pattern of errors in our study. One possibility is that while increasing the top-down allocentric processing demand of the task the demand for left-right egocentric processing was attenuated. In individuals with spatial neglect, evidence suggests that task demands (Baylis, Baylis, & Gore, 2004) and the stimuli used to test for neglect (Tipper & Behrmann, 1996) can significantly impact how deficits manifest with respect to different reference frames (Fink, Marshall, Weiss, Toni, & Zilles, 2002). According to this task-relevance view of neglect, the foci and width of spatial attention is guided by task requirements, which in turn define the perceptual representation of the space (Baylis et al., 2004). Perhaps requiring participants to explicitly report the egocentric locations of lines, thereby increasing the egocentric demand of the task, could induce errors consistent with egocentric processing after the right SMG stimulation. Another possibility is that a blocked design of the task, where viewer-centered line locations are kept constant within a block, would focus attention to viewer-centered left or right in a block, and might induce bias consistent with egocentric processing after SMG stimulation. These hypotheses remain to be tested in future studies. We also considered the more straightforward possibility that the location within the right SMG that we chose to target with rTMS may not be consistently involved in egocentric processing across all our participants, which could explain the negative findings. In future studies, the use of neuroimaging to localize individualized targets of stimulation will address this potential issue (Oliver, Bjoertomt, Driver, Greenwood, & Rothwell, 2009).
In addition to the effects of rTMS, the findings related to our participants’ intrinsic spatial biases were interesting and corroborated with several prior studies. Our participants were least accurate on the bisection trials compared to the two non-bisection trial types. On bisection trials, participants were more accurate when lines were presented to the viewer-centered left compared to their right (Figure 4). These results are most likely linked to the functional lateralization of spatial attention to the right hemisphere, favoring the viewer-centered left space, as demonstrated in a recent fMRI study using a line bisection judgment task similar to the one we used (Zago et al., 2017). Additionally, among the non-bisection trial types, participants were more accurate on the central than on the peripheral trials. Because of the lateralized presentation of lines with fixation at the center, we think that peripheral visual resolution drop off that typically occurs with increasing stimulus eccentricity (Hamilton, Stark, & Coslett, 2010; Johnson & Leibowitz, 1979), critically affected accuracy in peripheral trials (Larson & Loschky, 2009).
While our findings provide a better understanding of spatial processing in different reference frames, our methods differentiating egocentric and allocentric processing may also lead to better assessment for clinical use. The lateralized landmark task is a novel implementation of the landmark task that allowed us to better discriminate between allocentric and egocentric spatial processing systems than done previously using the conventional landmark task (Oliveri & Vallar, 2009). With minor modifications to the task, such as increasing the timing of stimulus display, it can be readily implemented in assessing patients with spatial neglect for allocentric deficits. This will address the need that was highlighted in our prior work (Shah et al., 2013) for sensitive neuropsychological assessments for mild to moderate allocentric neglect in the clinical population. Mild to moderate deficits are often under-diagnosed using current paper-and-pencil assessments, but may still give rise to functional impairments, underscoring the need for robust behavioral measures of spatial processing.
One limitation of the current study is a relatively small sample size (N=11). While we believe the sample size to be appropriate for a proof-of-concept study, larger future studies would be useful to lend additional support for our findings. In conclusion, our study findings add to the growing body of evidence and further clarify the role of right STG in intact allocentric processing using a novel, lateralized version of the landmark task. Our findings lend additional support for the right hemispheric dominance and effects of eccentricity in healthy visuospatial processing. While the current study investigated individual brain areas with rTMS, combining rTMS with functional neuroimaging will afford further insights into how these individual brain areas interact during tasks requiring both allocentric and egocentric spatial processing.
Supplementary Material
Highlights.
The role of superior temporal gyrus in healthy spatial processing remains less clear.
Lateralized landmark task was designed to accentuate allocentric task demand.
Inhibitory repetitive transcranial magnetic stimulation (rTMS) paradigm was used.
Disruption of the right STG but not SMG induced stimulus-centered judgment errors.
Our results confirm STG’s involvement in healthy allocentric spatial processing.
Acknowledgments
The current study was funded by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation and the NIH/NINDS. We will like to thank the members of the Laboratory for Cognition and Neural Stimulation (LCNS), particularly Felix Gervits, Olufunsho Faseyitan, Caitlin Breslin and Gabriella Garcia for helping with participant recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson B. A mathematical model of line bisection behaviour in neglect. Brain. 1996;119(Pt 3):841–850. doi: 10.1093/brain/119.3.841. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. J Rehabil Res Dev. 2006;43(3):337–346. doi: 10.1682/jrrd.2005.01.0015. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Baylis GC, Baylis LL, Gore CL. Visual neglect can be object-based or scene-based depending on task representation. Cortex. 2004;40(2):237–246. doi: 10.1016/s0010-9452(08)70119-9. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Ricci R, Lualdi M, Colombo MR. Perceptual and response bias in unilateral neglect: two modified versions of the milner landmark task. Brain Cogn. 1998;37(3):369–386. doi: 10.1006/brcg.1998.1003. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, Coslett HB. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62(5):749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- Chen P, Caulfield MD, Hartman AJ, O'Rourke J, Toglia J. Assessing viewer-centered and stimulus-centered spatial bias: The 3s spreadsheet test version 1. Appl Neuropsychol Adult. 2016:1–8. doi: 10.1080/23279095.2016.1220382. [DOI] [PubMed] [Google Scholar]
- Cicek M, Deouell LY, Knight RT. Brain activity during landmark and line bisection tasks. Front Hum Neurosci. 2009;3:7. doi: 10.3389/neuro.09.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Baylis GC, Goodrich SJ, Rafal RD. Axis-based neglect of visual shapes. Neuropsychologia. 1994;32(11):1353–1365. doi: 10.1016/0028-3932(94)00068-9. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127(Pt 10):2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, Dieterich M. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage. 2003;20(3):1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Toni I, Zilles K. Task instructions influence the cognitive strategies involved in line bisection judgements: evidence from modulated neural mechanisms revealed by fMRI. Neuropsychologia. 2002;40(2):119–130. doi: 10.1016/s0028-3932(01)00087-2. [DOI] [PubMed] [Google Scholar]
- Galati G, Lobel E, Vallar G, Berthoz A, Pizzamiglio L, Le Bihan D. The neural basis of egocentric and allocentric coding of space in humans: a functional magnetic resonance study. Exp Brain Res. 2000;133(2):156–164. doi: 10.1007/s002210000375. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A, Fruhmann Berger M, Tatagiba M, Karnath HO. The role of the right superior temporal gyrus in visual search-insights from intraoperative electrical stimulation. Neuropsychologia. 2006;44(12):2578–2581. doi: 10.1016/j.neuropsychologia.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA, Milner AD. Two distinct modes of control for object-directed action. Prog Brain Res. 2004;144:131–144. doi: 10.1016/s0079-6123(03)14409-3. [DOI] [PubMed] [Google Scholar]
- Grimsen C, Hildebrandt H, Fahle M. Dissociation of egocentric and allocentric coding of space in visual search after right middle cerebral artery stroke. Neuropsychologia. 2008;46(3):902–914. doi: 10.1016/j.neuropsychologia.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Stark M, Coslett HB. Increased effect of target eccentricity on covert shifts of visual attention in patients with neglect. Cortex. 2010;46(1):68–76. doi: 10.1016/j.cortex.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M, Kramer-McCaffery T, Dow L, Murphy PJ, Gilchrist ID. Categorisation of 'perceptual' and 'premotor' neglect patients across different tasks: is there strong evidence for a dichotomy? Neuropsychologia. 2002;40(8):1387–1395. doi: 10.1016/s0028-3932(01)00202-0. [DOI] [PubMed] [Google Scholar]
- Harvey M, Milner AD, Roberts RC. An investigation of hemispatial neglect using the Landmark Task. Brain Cogn. 1995;27(1):59–78. doi: 10.1006/brcg.1995.1004. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Mechanisms underlying hemispatial neglect. Ann Neurol. 1979;5(2):166–170. doi: 10.1002/ana.410050210. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25(12):3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38(1):93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Leibowitz HW. Practice effects for visual resolution in the periphery. Percept Psychophys. 1979;25(5):439–442. doi: 10.3758/bf03199854. [DOI] [PubMed] [Google Scholar]
- Khurshid S, Trupe LA, Newhart M, Davis C, Molitoris JJ, Medina J, Hillis AE. Reperfusion of specific cortical areas is associated with improvement in distinct forms of hemispatial neglect. Cortex. 2012;48(5):530–539. doi: 10.1016/j.cortex.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M. Mechanisms of Unilateral Neglect. In: Jeannerod M, editor. Advances in Psychology 45: Neurophysiological and Neuropsychological Aspects of Spatial Neglect. North-Holland: Elsevier Science; 1987. p. 69. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RB. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software. 2017;82(1):1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Larson AM, Loschky LC. The contributions of central versus peripheral vision to scene gist recognition. J Vis. 2009;9(10):6, 1–16. doi: 10.1167/9.10.6. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE. Dissociation between egocentric and allocentric visuospatial and tactile neglect in acute stroke. Cortex. 2008;44(9):1215–1220. doi: 10.1016/j.cortex.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt ME. Performance consistency of normal observers in forced-choice tachistoscopic visual line bisection. Neuropsychologia. 2001;39(10):1065–1076. doi: 10.1016/s0028-3932(01)00044-6. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Slater J. Centripetal versus centrifugal bias in visual line bisection: focusing attention on two hypotheses. Front Biosci. 2000;5:D58–71. doi: 10.2741/a496. [DOI] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, Hillis AE. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J Cogn Neurosci. 2009;21(11):2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Postma P, Moutsopoulou K, Nimmo-Smith I, Marcel A, Walsh V. TMS over right posterior parietal cortex induces neglect in a scene-based frame of reference. Neuropsychologia. 2006;44(7):1222–1229. doi: 10.1016/j.neuropsychologia.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Van der Lubbe RH, Ramsey NF, Postma A. Interactions between ego- and allocentric neuronal representations of space. Neuroimage. 2006;31(1):320–331. doi: 10.1016/j.neuroimage.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Oliver R, Bjoertomt O, Driver J, Greenwood R, Rothwell J. Novel 'hunting' method using transcranial magnetic stimulation over parietal cortex disrupts visuospatial sensitivity in relation to motor thresholds. Neuropsychologia. 2009;47(14):3152–3161. doi: 10.1016/j.neuropsychologia.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Vallar G. Parietal versus temporal lobe components in spatial cognition: Setting the mid-point of a horizontal line. J Neuropsychol. 2009;3(Pt 2):201–211. doi: 10.1348/174866408X388197. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Pouget A, Driver J. Relating unilateral neglect to the neural coding of space. Curr Opin Neurobiol. 2000;10(2):242–249. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2017 from https://www.R-project.org/
- Shah PP, Spaldo N, Barrett AM, Chen P. Assessment and functional impact of allocentric neglect: a reminder from a case study. Clin Neuropsychol. 2013;27(5):840–863. doi: 10.1080/13854046.2013.783120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Behrmann M. Object-centered not scene-based visual neglect. J Exp Psychol Hum Percept Perform. 1996;22(5):1261–1278. doi: 10.1037//0096-1523.22.5.1261. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133(Pt 3):880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Zago L, Petit L, Jobard G, Hay J, Mazoyer B, Tzourio-Mazoyer N, Mellet E. Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychologia. 2017;94:75–83. doi: 10.1016/j.neuropsychologia.2016.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.