Abstract
IMPORTANCE
Recent research on addiction-related memory processes suggests that protracted extinction training following brief cue-elicited memory retrieval (ie, retrieval-extinction [R-E] training) can attenuate/eradicate the ability of cues to elicit learned behaviors. One study reported that cue-elicited craving among detoxified heroin addicts was substantially attenuated following R-E training and through 6-month follow-up.
OBJECTIVE
To build on these impressive findings by examining whether R-E training could attenuate smoking-related craving and behavior.
DESIGN, SETTING, AND PARTICIPANTS
This prospective, mixed-design, human laboratory randomized clinical trial took place between December 2013 and September 2015. Participants were recruited in Charleston, South Carolina. Study sessions took place at the Medical University of South Carolina. The participants were 168 screened volunteer smokers, of whom 88 were randomized; 72 of these 88 participants (81.8%) attended all the follow-up sessions through 1 month. The primary eligibility criteria were current nicotine dependence (DSM criteria), smoking 10 or more cigarettes per day, and a willingness to attempt smoking cessation.
INTERVENTIONS
Participants were randomly assigned to receive either smoking-related memory retrieval followed by extinction training (the R-E group) or nonsmoking-related retrieval followed by extinction training (the NR-E group).
MAIN OUTCOMES AND MEASURES
Primary outcomes were cue-elicited craving and physiological responding to familiar and novel cues in the R-E group vs the NR-E group over a 1-month follow-up period. Secondary outcomes were smoking-related behaviors.
RESULTS
A total of 44 participants were randomly assigned to the R-E group (mean age, 48.3 years; 72.7% male); a total of 44 participants were randomly assigned to the NR-E group, with 43 attending at least 1 training session (mean age, 46.7 years; 55.8% male). The mean craving response to both familiar and novel smoking cues was significantly lower for participants in the R-E group than for participants in the NR-E group at 1-month follow-up (for both cue types: t1225 = 2.1, P = .04, d = 0.44, and Δ = 0.47 [95% CI, 0.04–0.90]). The mean numbers of cigarettes smoked per day at 2 weeks and 1-month were significantly lower for the R-E group than for the NR-E group (treatment main effect: F1,68 = 5.4, P = .02, d = 0.50, and Δ = 2.4 [95% CI, 0.4–4.5]). Significant differences in physiological responses, urine cotinine level, number of days abstinent, lapse, and relapse were not observed between groups (all between P = .06 and .75).
CONCLUSIONS AND RELEVANCE
Retrieval-extinction training substantially attenuated craving to both familiar and novel smoking cues and reduced the number of cigarettes smoked per day by participants 1 month after treatment relative to extinction training alone. Between-group differences were not observed for physiological responses, cotinine level, number of days abstinent, relapse, or lapse. In summary, R-E training is a brief behavioral treatment that targets smoking-related memories and has the potential to enhance relapse prevention.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT02154685
The role of learning and memory processes in addiction has been well established in both theory and research.1–6 A fundamental source of this learning occurs when cues chronically paired with drug reward gradually acquire the capacity to elicit a range of conditioned responses, such as craving and physiological reactivity. In the case of nicotine-reinforced smoking addiction, individuals trying to quit smoking commonly report that cue-elicited craving plays a central role in smoking lapses.7–11 Smokers with increased vulnerability to cue-elicited craving and those who experience greater reactivity to cues are less likely to quit successfully.12–15 These findings, together with clinical studies demonstrating that first-line cessation medications have only modest effects on cue-elicited cigarette craving,16–19 suggest that there is a need to develop treatments aimed at reducing cue-induced craving and the associated relapse risk.
Initial efforts to address cue-elicited craving and reactivity were based on human and animal studies of extinction.20,21 Extinction training involves protracted, unreinforced exposure to cues that control conditioned responding, the result of which is a diminution of cue-elicited responding. In the case of addictive behavior, extinction-based therapy became known as cue exposure therapy and consisted of repeated exposure to drug-associated cues in the absence of drug reward. Although cue exposure therapy was intuitively appealing, efficacy studies have indicated minimal utility,22 possibly because extinction training does not alter the original learning but, rather, results in the development of inhibitory learning that suppresses responding.20,21,23–27 Importantly, the ability of the inhibitory learning to oppose the original learning and suppress responding is constrained by a number of factors, including the passage of time (ie, spontaneous recovery), the presence of novel drug cues or contexts (ie, renewal), or the occurrence of drug reward following drug administration (ie, reinstatement).28–30 Recent neuroscience research on memory reconsolidation suggests that there may be a way to induce enduring changes in the memories that support responding to cues.31,32
Reconsolidation refers to a retrieval-induced, time-limited “window” of opportunity during which memories are amenable to pharmacological or behavioral alteration. Pharmacological agents aimed at attenuating reconsolidation of fear-based and drug-reinforced learning have shown promise in both animals33–39 and humans.40–46 A recently developed behavioral approach to memory modulation involves the strategic administration of extinction training during the re-consolidation window.47,48 This retrieval-extinction [R-E] training is presumed to result in the updating of the original memory with new information that is incongruent with the established cue-drug contingency (ie, drug cues no longer predict pharmacological reward). Because R-E training directly targets memories for drug-related learning, it is assumed to produce more enduring changes in drug-reinforced behavior than can be achieved with conventional extinction.
To our knowledge, a recent study49 in Science provides the only published human study examining the relevance of R-E training on clinically relevant addictive behavior. In that clinical translational study, Xue et al49 showed that, in heroin addicts, craving and cue-reactivity to heroin cues could be pro foundly impaired by 2 sessions of R-E training; importantly, this effect was still evident 6 months after the intervention, indicating a nearly complete absence of spontaneous recovery. The primary goal of the present study was to replicate (partially) and extend the findings of Xue et al49 by evaluating the effects of R-E training on craving, physiological reactivity, and smoking behavior in a sample of nicotine-dependent cigarette smokers. The present study extended the study by Xue et al49 by examining the effects of R-E training on drug use behavior (smoking behavior) and by examining the generalizability of treatment effects to novel drug cues.
The primary hypothesis was that R-E training would produce lower cue-elicited craving and physiological reactivity (during follow-up test sessions) than conventional extinction training (ie, nonsmoking-related retrieval–extinction [NR-E] training). Secondary hypotheses were that (1) R-E training would attenuate smoking (eg, reduce the number of cigarettes smoked per day) and/or increase latency to smoking lapse/relapse over the 1-month follow-up period, and (2) the effects of R-E training would generalize to novel cues.
Methods
Participants
Participants were treatment-seeking cigarette smokers from Charleston, South Carolina, who were recruited through media advertisement, fliers, and referrals from friends (Trial Protocol in Supplement 1). A brief telephone screening assessed participant suitability via inclusion/exclusion criteria, and qualified individuals underwent a more detailed assessment at the Medical University of South Carolina. Primary inclusion criteria included a willingness to attempt a 3-day cessation without cessation aids, smokeless tobacco, or electronic cigarettes; meeting the DSM-IV criteria for current nicotine dependence; and smoking 10 or more cigarettes per day for 3 years or more. Participants were excluded if they were pregnant, met diagnostic criteria for substance dependence other than nicotine in the past 60 days (abstinence verified via breathalyzer and urine drug screening test during the baseline, 2 R-E or NR-E training sessions, and a 24-hour follow-up session), or met diagnostic criteria for current/active (untreated) psychological disorders (additional details of exclusion criteria can be found in the eAppendix in Supplement 2). The trial was approved by the institutional review board of the Medical University of South Carolina and the protocol appears in Supplement 1. All participants provided written informed consent prior to the study.
Procedure
Figure 1 provides a diagrammatic summary of the study design and procedures. Participants attended a baseline session, 2 R-E or NR-E training sessions on consecutive days, and 24-hour, 2-week, and 1-month postintervention follow-up test sessions. After providing consent at the baseline session, participants provided demographic and clinical information. Smoking abstinence was assessed using self-report verification (ie, the timeline follow-back method50) and breath carbon monoxide (CO) assessment, with overnight abstinence confirmed if the participant’s breath CO level was 10 ppm or less. Abstinence from alcohol and other drugs was confirmed via a breathalyzer indicating a breath alcohol concentration of 0.0% and negative test results from a urine drug screen, respectively. The samples from the urine drug screens were also collected to establish cotinine levels. Participants then completed a baseline cue-reactivity assessment. Precue and postcue assessments of craving (using the self-report Craving Questionnaire51), negative affect (using a modified version of the self-report Mood Form52), heart rate (HR), and blood pressure (BP) were collected (Figure 1). Participants were reminded that abstinence was required and monetarily compensated during the ensuing 3-day period in which the 2 R-E or NR-E training sessions and the 24-hour test session would occur.
Figure 1. Study Design and Procedure.
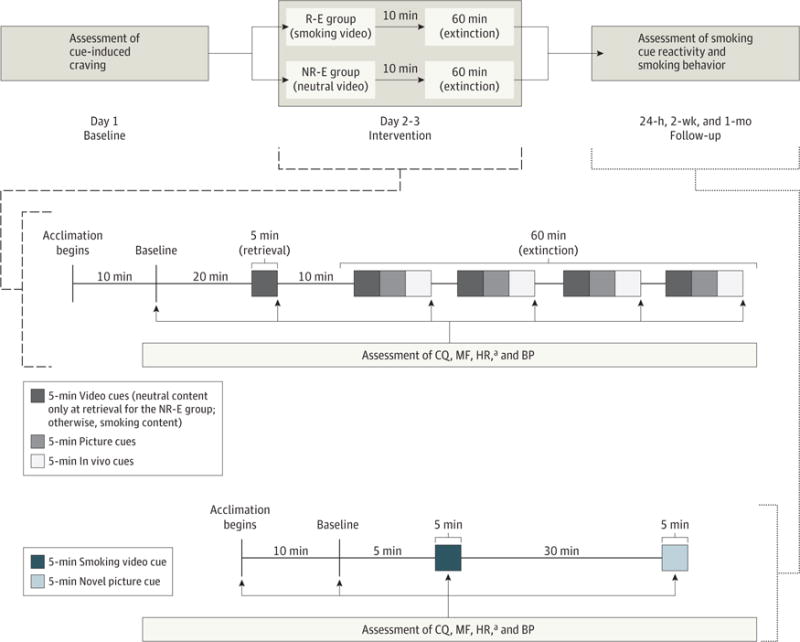
The upper portion indicates the overall study design. The middle portion indicates the assessment of the Craving Questionnaire (CQ), the Mood Form (MF), heart rate (HR), and blood pressure (BP) at baseline, after the retrieval video (the video had smoking content for the retrieval-extinction [R-E] group but neutral, nonsmoking content for the nonsmoking-related retrieval–extinction [NR-E] group), and postextinction cues (all cues contained smoking content) during the R-E or NR-E training sessions. The lower portion indicates CQ, MF, HR, and BP assessments during the 3 follow-up test sessions.
aTaken during the first 50 seconds of cue exposure; all other assessments were taken at the end of a particular cue series.
At the 2 R-E or NR-E training sessions, participants underwent a breath CO assessment, a urine drug screen, and breathalyzer test after arriving at the Medical University of South Carolina to assess compliance with abstinence. Upon verification of abstinence from all substances at the first R-E or NR-E training session, participants were randomly assigned to either the R-E group or the NR-E group. Only the 2 R-E or NR-E training sessions involved a retrieval-cue presentation (5-minute smoking retrieval videos for the R-E group and neutral videos for the NR-E group) during the cue-reactivity assessment (Figure 1). Precue and postcue assessments of the Craving Questionnaire, Mood Form, HR, and BP of each participant were collected during the cue-reactivity assessment.
Participants completed test sessions (3) at 24 hours, 2 weeks, and 1 month after the second R-E or NR-E training session (Figure 1). Participants provided breath CO, urine drug screen, and breathalyzer assessments at test sessions (cotinine level was not assessed at the 24-hour test session) and engaged in cue-reactivity assessments involving the presentation of familiar smoking video cues and novel smoking picture cues. Measures of the Craving Questionnaire, the Mood Form, HR, and BP were collected at the same times as during the baseline and training sessions. Data on the secondary smoking outcomes of the number of cigarettes smoked per day, the percentage reduction in number of cigarettes smoked from baseline, the number of days abstinent from cigarettes, lapse, and relapse were collected between the 24-hour and 2-week follow-ups and between the 2-week and 1-month follow-ups (lapse and relapse are defined in the eAppendix in Supplement 2).
The primary outcomes were craving, negative affect, blood pressure, and heart rate. The secondary outcomes included number of cigarettes smoked per day, breath CO level, percentage reduction in number of cigarettes smoked from baseline, number of days abstinent from cigarettes, lapse, and relapse.
Sample Size Estimation and Randomization
The study sample size estimation was based on the primary outcome of cue-elicited craving during the test sessions, as well as on the secondary smoking-related outcome of the number of cigarettes smoked per day. The sample size of 88 randomized participants was determined to provide 80% power, at α ≤ .05, to detect a minimum effect size of 0.66 for the continuous outcomes, even in the wake of higher-than-expected study attrition. Stratified urn randomization was used to assign participants to either the R-E group or the NR-E group.53 Urn variables were sex and level of nicotine dependence (determined using the Fagerström Test of Nicotine Dependence54). Nicotine dependence was stratified accordingly (a Fagerström Test of Nicotine Dependence score of ≤5 vs >5).
Statistical Analysis
Linear mixed effects models, generalized estimating equation models, and correlational analyses were used to analyze data. Although not powered to evaluate group differences in abstinence and abstinence-related milestones (lapse and relapse variables noted above), we preliminarily examined these outcomes. Latency to smoke was investigated using Cox proportional hazard models where the first day following the abstinence period was considered the beginning of the risk period. Additional details pertaining to data management and analysis are available in the eAppendix in Supplement 2.
Results
Participant Characteristics
A CONSORT flow diagram is shown in Figure 2. Of the 88 individuals randomly assigned to receive either R-E or NR-E training, 87 (98.9%) attended at least 1 training session, and 76 (86.4%) attended at least 1 test session (72 [81.8%] attended all test sessions). There was no difference in retention to test sessions between treatment groups ( , P = .53). Of the participants who remained abstinent during the baseline session and the 3-day window (2 R-E or NR-E training sessions and a 24-hour test session), there were significant reductions in CO level throughout (mean [SD] level at baseline session: 6.1 [2.4] ppm; at first R-E or NR-E training session: 5.6 [2.6] ppm; at second R-E or NR-E training session: 3.0 [1.6] ppm; at 24-hour test session: 2.8 [1.7] ppm; F3,222 = 74.6, P < .001) with no significant treatment-by-time effect (P = .50). Although follow-up sessions were planned 2 weeks after and 1 month after completion of treatment, variation in time between follow-up sessions occurred. The median time to the 2-week follow-up session was 14 days (range, 12–21 days), and the median time between the 2-week and 1 month follow-up session was 14 days (range, 10–32 days). There were no treatment assignment differences between the 2 study follow-up durations (2-week visit: Z = −0.7, P = .50 and 1-month visit: Z = −0.1, P = .89). Baseline demographic and clinical characteristics are reported for treatment groups in the Table.
Figure 2. CONSORT Flow Diagram.
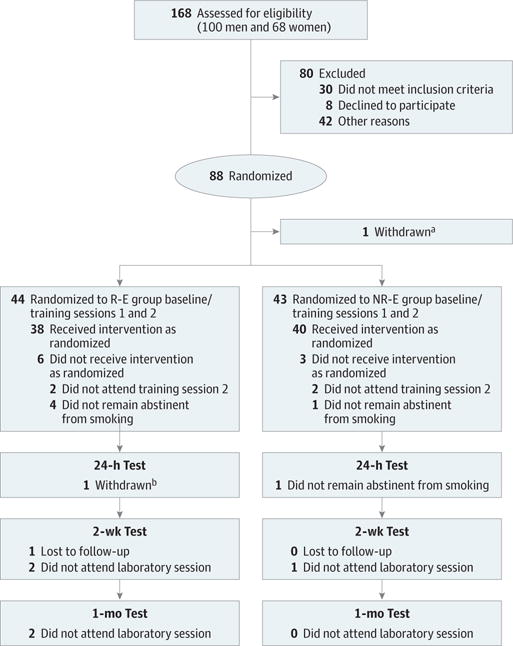
Other reasons for exclusion prior to randomization included not attending the randomization session, data integrity issues, and enrollment in the study after recruitment goal was achieved. NR-E indicates nonsmoking-related retrieval–extinction; R-E, retrieval-extinction.
aOne participant was withdrawn from the trial after randomization owing to cotinine levels indicating that she was a nonsmoker.
bOne participant was withdrawn owing to reporting that he smoked in between training sessions.
Table.
Demographic and Clinical Characteristics of Treatment Groupsa
| Characteristic | Participants, No. (%) | Statisticb | P Valuec | |
|---|---|---|---|---|
| Group R-E (n = 44) |
Group NR-E (n = 43) |
|||
| Age, mean (SD), y | 48.3 (12.5) | 46.7 (12.8) | t = 0.56 | .57 |
| Sex | ||||
| Male | 32 (72.7) | 24 (55.8) | χ2 = 2.71 | .10 |
| Female | 12 (27.3) | 19 (44.2) | ||
| Race | ||||
| Black | 23 (52.3) | 23 (53.5) | χ2 = 0.13 | .91 |
| White or otherd | 21 (47.7) | 20 (46.5) | ||
| Education | ||||
| No HS completion | 3 (6.8) | 4 (9.3) | ||
| HS graduate or equivalent | 15 (34.1) | 13 (30.2) | χ2 = 0.27 | .87 |
| Some college or college graduate | 26 (59.1) | 26 (60.5) | ||
| Employed | ||||
| Yes | 19 (43.2) | 13 (30.2) | χ2 = 1.57 | .21 |
| No | 25 (56.8) | 30 (69.8) | ||
| Annualhousehold income, $ | ||||
| ≤20 000 | 18 (40.9) | 24 (55.8) | χ2 = 1.94 | .16 |
| >20 000 | 26 (59.1) | 19 (44.2) | ||
| Marital status | ||||
| Married | 12 (27.3) | 12 (27.9) | χ2 = 0.004 | .95 |
| Othere | 32 (72.7) | 31 (72.1) | ||
| Age at first cigarette smoked, mean (SD), y | 15.0 (4.4) | 13.9 (4.8) | t = 1.06 | .29 |
| No. of cigarettes smoked/d,f mean (SD) | 16.9 (6.7) | 16.0 (7.8) | t = 0.54 | .59 |
| CO level, mean (SD), ppm | 6.2 (2.3) | 6.2 (2.7) | t = 0.27 | .79 |
| Cotinine level, mean (SD), μg/L | 871.1 (613.7) | 1067.4 (584.2) | t = 1.53 | .13 |
| History of smoking, mean (SD), y | 28.1 (12.1) | 27.8 (13.3) | t = 0.09 | .93 |
| No. of past quit attempts, mean (SD) | 5.6 (18.8) | 5.7 (8.4) | t = 0.01 | .99 |
| FTND totalscore, mean (SD) | 4.9 (2.0) | 4.8 (1.6) | t = 0.24 | .81 |
| No. of MINI diagnoses | ||||
| 0 | 7 (15.9) | 10 (23.3) | χ2 = 1.66 | .44 |
| 1 | 9 (20.5) | 5 (11.6) | ||
| ≥2 | 28 (63.6) | 28 (65.1) | ||
Abbreviations: CO, carbon monoxide; FTND, Fagerström Test of Nicotine Dependence; HS, high school; MINI, Mini-International Neuropsychiatric Interview; NR-E, nonsmoking-related retrieval–extinction; R-E, retrieval-extinction.
SI conversion factor: To convert cotinine to nanomoles per liter, multiply by 5.675.
All assessments were collected at baseline.
Group differences were assessed via t tests (continuous) or χ2 tests (categorical). For all t tests, df = 85, and for all χ2 tests, df = 1.
From 2-tailed tests, with α < .05.
Includes persons who identified as white, Asian, Native American, Alaskan native, or Native Hawaiian or other Pacific Islander.
Identifies persons never married, separated, divorced, or widowed.
Data on the number of cigarettes smoked per day are reported for the past 2 weeks prior to the baseline session (assessed via the timeline follow-back method).
Craving and Negative Affect
Craving decreased significantly across test sessions (Figure 3A; final models adjusted for baseline craving, number of days between follow-up sessions, years of regular smoking, and sex; F4,287 = 20.7, P < .001) while increasing within sessions following exposure to smoking cues (F16,219 = 2.4, P = .001). The overall main effect of treatment assignment on craving response during the test sessions was nonsignificant (F1,81 = 2.3, P = .13), although craving response to both the smoking video cues and the novel picture cues were significantly attenuated in the R-E group compared with the NR-E group during the last test session (for both cue types, t1225 = 2.1, P = .04, d = 0.44, and Δ = 0.47 [95% CI, 0.04–0.90]). Negative affect decreased significantly across test sessions (adjusted for baseline negative affect and number of days between follow-up sessions; F4,291 = 7.9, P < .001). However, there was not a significant overall main effect of treatment on negative affect during test sessions (F1,83 = 1.8; P = .19).
Figure 3. Craving- and Smoking-Related Outcomes by Treatment Group.
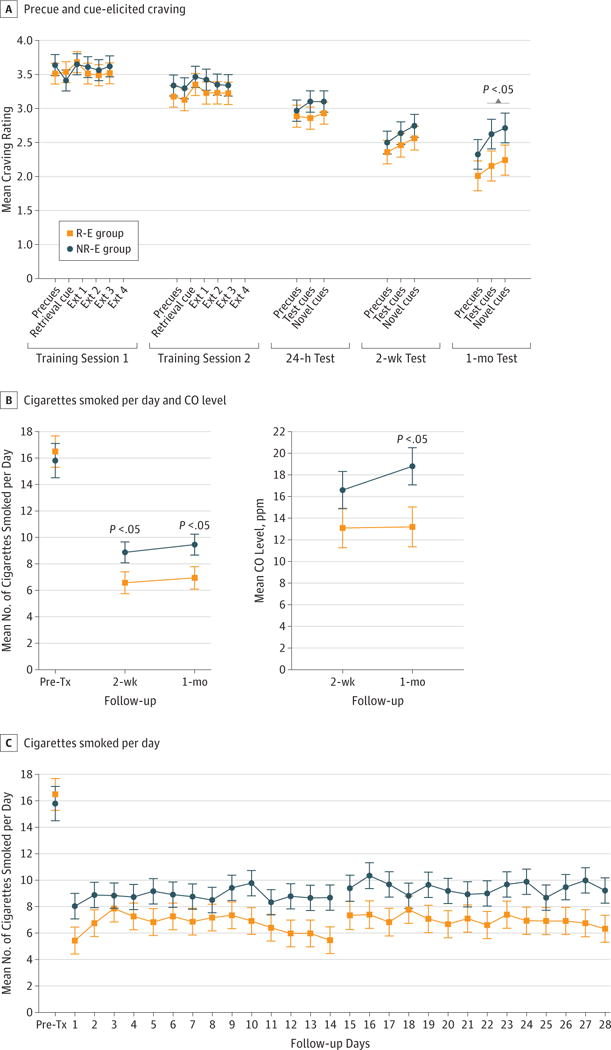
Precue and cue-elicited craving at the 2 retrieval-extinction (R-E) or nonsmoking-related retrieval–extinction (NR-E) training sessions and 3 follow-up test sessions (A), the mean number of cigarettes smoked per day and carbon monoxide (CO) level at 2-week and 1-month follow-up sessions (“Pre-Tx” indicates the mean number of pretreatment cigarettes smoked per day over the 2 weeks prior to study engagement) (B), and the mean number of cigarettes smoked per day before treatment and on the 14 days prior to the 2-week test session (follow-up days 1–14) and the 1-month test session (follow-up days 15–28) (C). Error bars indicate SE. “Precues” indicate craving prior to any cue presentation; “Retrieval cue” indicates craving in response to the smoking-related (R-E group) or neutral-related (NR-E group) retrieval video; and “Ext 1, 2, 3, and 4” indicate each of the four 15-minute extinction sequences.
Physiological Responses
Blood pressure increased significantly within each training and test session with presentation of the cues (systolic BP [SBP]: F16,219 = 4.3, P < .001; diastolic BP [DBP]: F16,219 = 6.6, P < .001) but did not change significantly across test sessions (adjusted for baseline BP; P = .95 and .45 for SBP and DBP, respectively). There was a marginally significant main effect of treatment on SBP (F1,82 = 3.8, P = .06), with higher SBP in the R-E group vs the NR-E group during follow-up sessions. However, there was no main effect of treatment on DBP (F1,82 = 1.1; P = .16). There was also no difference in BP during the last test session that paralleled the craving response (all between P = .54 and .92). Although HR decreased significantly within each session (F16,1217 = 14.2, P < .001), there was a significant increase in HR across sessions (adjusted for baseline HR; F4,287 = 8.5, P < .001). There was no effect of treatment on HR during test sessions (F1,72 = 0.5, P = .47), including the final test session (familiar cues: t415 = 1.5, P = .12; novel cues: t415 = 0.9, P = .38).
Smoking Behavior
Following the 2 training sessions, participants in the R-E group reported smoking fewer cigarettes per day relative to those in the NR-E group (Figure 3B and C; the treatment main effect was F1,68 = 5.4, P = .02, d = 0.50, and Δ = 2.4 [95% CI, 0.4–4.5] after adjusting for number of cigarettes smoked per day at baseline, number of years of regular smoking, sex, and baseline negative affect). In addition, there was trend-level evidence that a greater percentage of participants in the R-E group (51.5%) than in the NR-E group (25.6%) achieved a 60% reduction in smoking (from baseline) during follow-up (risk ratio, 1.62 [95% CI, 0.98–2.67], P = .06; at 2-week and 1-month follow-up time points, P = .18 and .04, respectively). No differences were found for 50% and 75% reductions in cigarettes smoked during follow-up (50% reduction: risk ratio, 1.35 [95% CI, 0.93–1.96], , P = .12; 75% reduction: risk ratio, 1.80 [95% CI, 0.84–3.84], , P = .13). Expired CO levels were significantly attenuated in the R-E group compared with the NR-E group at the 1-month test session (adjusting for baseline negative affect and race; t67 = 2.2, P = .03, d = 0.47; Figure 3B). Despite a significant reduction in the number of cigarettes smoked per day and in CO level for the R-E group relative to the NR-E group, differences in urine cotinine levels (adjusted for baseline cotinine levels), total number of days abstinent (adjusted for number of cigarettes smoked per day at baseline), and smoking lapse failed to reach significance between the R-E group and the NR-E group (all between P = .72 and .75). In addition, the 2 groups did not differ on measures of smoking relapse (7-day criterion: hazard ratio, 0.88 [95% CI, 0.55–1.44], P = .38; 3- to 5-day criterion: hazard ratio, 1.42 [95% CI, 0.85–2.39], P = .19 [both adjusted for number of cigarettes smoked per day at baseline]; see eAppendix in Supplement 2 for definitions of relapse measures).
There were no significant moderating effects of craving on treatment for any of the smoking outcomes (all P > .15). However, higher cue-induced craving was correlated with greater negative affect (video cues: ρ = 0.18, P = .03; picture cues: ρ = 0.20, P = .02), higher HR (picture cues: ρ = 0.20, P = .02), and fewer abstinent days (video cues: ρ = −0.29, P < .001; picture cues: ρ = −0.31, P < .001) and was marginally correlated with higher CO level (picture cues: ρ = 0.14, P = .08). There were no additional significant correlations between cue-induced craving and negative affect, physiological measures, biochemical verification assessments, and smoking behavior (all between ρ = 0.01 and 0.13; all between P = .14 and .91).
Discussion
The primary findings of this study were that R-E training significantly reduced craving and the number of cigarettes smoked per day relative to massed extinction training without retrieval (ie, NR-E training). Importantly, the self-reported smoking findings were corroborated by assessment of CO levels. These findings are consistent with the reconsolidation hypothesis, which would assert that a very brief (5-minute) smoking cue video, followed shortly after by massed extinction, would result in the updating of the cue-drug contingency in memory and produce the observed behavioral outcomes. In summary, to our knowledge, this study is the first investigation to evaluate the effects of a brief R-E training procedure on clinically relevant smoking behavior, with craving and smoking reductions either emerging or maintained at 1-month follow-up, respectively.
The present findings are consistent with the seminal work by Monfils et al,47 which showed that R-E training produced reductions in conditioned fear that are resistant to spontaneous recovery, renewal, and reinstatement. Furthermore, the present findings are consistent with a growing body of positive human and animal laboratory studies,25,48,49,55–62 but inconsistent with several negative reports,43,45,63–66 the latter of which may be attributed to between-study methodological differences67 and interindividual differences.68–70 The clinical utility of R-E training has been examined in only 2 previously published studies. One was the previously noted study by Xue et al49 and the other71 used R-E training with spider phobics and found significant clinical benefits in both the R-E training and control groups. However, the equivalent outcomes were likely attributable to either a failure to induce reconsolidation or the occurrence of implicit fear reactivation in both groups.
The present study extended the findings of Xue et al49 to nicotine-dependent smokers, documenting attenuated smoking cue-elicited craving at 1-month follow-up but not at earlier test sessions. The self-reported number of cigarettes smoked per day was significantly lower in the R-E group at both the 2-week and 1-month follow-up test sessions. We also observed significant reductions in CO level at 1-month follow-up only. The contrasting delayed vs immediate effects of R-E training on craving vs smoking, respectively, was somewhat unexpected. However, one possible explanation pertains to the control group receiving extensive massed cue exposure, which may have resulted in the accrual of considerable extinction-related inhibition. It is possible that the extinction effects in the NR-E group persisted for approximately 2 weeks, thereby making it difficult to detect craving differences between the groups. However, with the passage of sufficient time and the cumulative effects of nicotine exposure via smoking (analogous to drug-primed reinstatement), the influence of extinction-related inhibition eroded in the NR-E group, while the effects of memory updating, which are known to be resistant to spontaneous recovery and drug-primed reinstatement, persisted in the R-E group. The net result of these 2 divergent processes was the emergence of differential craving at the 1-month test session. By contrast, the more immediate occurrence of group differences in smoking behavior could have been due to the relatively weak effects of extinction-related inhibition in the control group vs the relatively robust effects of updating in the R-E group.
The present study is the first to demonstrate the generalizability of R-E training effects in a clinical sample. Specifically, we observed attenuated cue-elicited craving in response to both familiar and novel cues at 1-month follow-up. Together with R-E training effects on craving, the attenuation of smoking behavior is notable and impressive. Importantly, smoking behavior was reduced even at 1-month follow-up, with a trend toward a higher percentage of smokers in the R-E group reducing their cigarette intake by 60% relative to the NR-E group. This suggests the possibility that R-E training endows participants with the ability to resist “reinstatement” of smoking and that R-E training may confer an advantage over cue exposure/extinction training in preventing relapse to smoking. Collectively, the results of this study suggest that R-E training may have the potential for use as an aid to smoking cessation.
The present study also sheds light on the issue of boundary conditions, especially as related to the effectiveness of re-consolidation manipulations on remote memories. Although a number of investigators suggest that remote memories are not amenable to alteration,37,72,73 our data highlight the possibility that memories resulting from innumerable drug-cue pairings over many years may be amenable to updating via R-E training. Thus, the present data foster a hope that memories relevant to addictive behavior may be altered to achieve desirable clinical outcomes.
Despite the noteworthy findings of the present study, there were some unexpected findings. First, the absence of a between-group difference in urine cotinine levels was unexpectedly inconsistent with the findings regarding craving, number of cigarettes smoked per day, and CO level. However, it is likely that urine cotinine level is not sufficiently sensitive to detect group differences in the number of cigarettes smoked per day, especially since urine cotinine level has been shown to be only 75% accurate in distinguishing between smokers and abstainers (across 10 studies74). Second, Xue et al49 reported significantly attenuated cue-induced SBP among participants in their retrieval group, whereas we found the opposite (although marginal) effect. This and other discrepant findings between the 2 studies could be explained by considerable methodological differences. For instance, the heroin addicts in Xue et al49 were in an inpatient hospital setting over the course of follow-up and, therefore, were drug-free, whereas the participants in our study could and did smoke throughout follow-up.
Limitations
Among our study limitations, the relatively short follow-up period stands out. The impressive attenuation of cue-elicited craving and smoking behavior appears as though it may have persisted beyond the 1-month follow-up window. Thus, it would be important to determine whether R-E training’s effects amplify and endure beyond the time frame observed here. We were also not sufficiently powered to examine a full range of smoking outcomes in the present study. A larger sample might have provided the additional power to detect group differences in relapse milestones for which we observed marginal trends. Although double-blinding would have been an attractive design feature, the blinding of participants would have been unachievable, and the blinding of study staff would have been very challenging and highly vulnerable to penetration. Finally, we did not assess motivation to quit at study completion and, therefore, were unable to determine whether it was altered by R-E training.
In addition to addressing these limitations, future research should use imaging methods to explore the effects of R-E training on the neural circuits implicated in craving and smoking outcomes. Future research might also examine the efficacy of R-E training when used as part of a multicomponent intervention consisting of both behavioral (eg, cognitive-behavioral therapy) and pharmacotherapy elements. The ease with which R-E training is administered would make it an especially good candidate for use with other interventions. Retrieval-extinction training could be easily adapted to treat other substance use disorders or anxiety disorders (eg, posttraumatic stress disorder).
Conclusions
To our knowledge, this study is the first investigation to evaluate the effects of R-E training on clinically relevant smoking behavior. We observed an impressive attenuation of cue-elicited craving and smoking in the R-E group through 1-month follow-up. In addition, R-E intervention’s effects generalized to novel smoking cues. The absence of between-group differences in cotinine level, number of days abstinent, and lapse and relapse variables was somewhat unforeseen, although we were not powered to evaluate group differences for most of these outcomes. In summary, the present study provides initial, compelling evidence that a brief reconsolidation-based intervention can attenuate smoking-related craving and behavior and points to the possibility that it may have utility as an aid to smoking cessation.
Supplementary Material
Key Points.
Question
Can protracted extinction training following brief cue-elicited memory retrieval attenuate smoking-related craving and behavior?
Findings
In this randomized clinical trial, retrieval-extinction training substantially attenuated the craving response to both familiar and novel smoking cues 1 month after treatment relative to extinction training alone. Although no between-group differences were observed for physiological responses, cotinine level, number of days abstinent, relapse, and lapse, between-group differences were observed for number of cigarettes smoked per day during follow-up, and a marginal difference was observed for a 60% smoking reduction at follow-up.
Meaning
A brief behavioral intervention that targets smoking-related memory processes can attenuate smoking-related craving and behavior, thereby suggesting the possibility that it might aid cessation.
Acknowledgments
Funding/Support: This research was funded by National Institute on Drug Abuse grant 5R21DA035993-02 (Dr Saladin, principal investigator), South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina, through National Institutes of Health grants UL1 RR029882 and UL1 TR000062.
Role of the Funder/Sponsor: The National Institute on Drug Abuse had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Mr Baker had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Carpenter, Froeliger, Saladin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Germeroth, Baker, Saladin.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Baker, Saladin.
Obtained funding: Saladin.
Administrative, technical, or material support: Froeliger, LaRowe, Saladin.
Study supervision: Saladin.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the paid research staff members Amanda Smith, BS, Nathan Forrester, BA, and Holly Campbell, BA (all from the Department of Health Sciences and Research, Medical University of South Carolina) for their invaluable contributions to this research (ie, data collection, management, and reduction).
Contributor Information
Lisa J. Germeroth, Department of Health Sciences and Research, Medical University of South Carolina, Charleston; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston.
Matthew J. Carpenter, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston; Hollings Cancer Center, Medical University of South Carolina, Charleston.
Nathaniel L. Baker, Department of Public Health Sciences, Medical University of South Carolina, Charleston.
Brett Froeliger, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston; Department of Neuroscience, Medical University of South Carolina, Charleston.
Steven D. LaRowe, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston; Mental Health Service Line, Ralph H. Johnson VA Medical Center, Charleston, South Carolina.
Michael E. Saladin, Department of Health Sciences and Research, Medical University of South Carolina, Charleston; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston.
References
- 1.Eikelboom R, Stewart J. Conditioning of drug-induced physiological responses. Psychol Rev. 1982;89(5):507–528. [PubMed] [Google Scholar]
- 2.Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96(4):609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman J, Packard MG. Memory systems and the addicted brain. Front Psychiatry. 2016;7:24. doi: 10.3389/fpsyt.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torregrossa MM, Taylor JR. Neuroscience of learning and memory for addiction medicine: from habit formation to memory reconsolidation. Prog Brain Res. 2016;223:91–113. doi: 10.1016/bs.pbr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. J Consult Clin Psychol. 1989;57(3):443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- 8.Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15(4):395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman S. Coping with temptations to smoke. J Consult Clin Psychol. 1984;52(2):261–267. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol. 1996;15(6):455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- 12.Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26(3):225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 13.Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. J Subst Abuse. 1989;1(4):393–405. [PubMed] [Google Scholar]
- 14.Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addict Behav. 1989;14(4):419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- 15.Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72(6):1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- 16.Gass JC, Wray JM, Hawk LW, Mahoney MC, Tiffany ST. Impact of varenicline on cue-specific craving assessed in the natural environment among treatment-seeking smokers. Psychopharmacology (Berl) 2012;223(1):107–116. doi: 10.1007/s00213-012-2698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68(2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Brandon TH, Drobes DJ, Unrod M, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218(2):391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlov IP. Conditioned Reflexes. New York, NY: Dover Publications; 1927. [Google Scholar]
- 21.Rescorla RA. Extinction. In: Bäckman L, von Hofsten C, editors. Psychology at the Turn of the Millennium: Cognitive, Biological, and Health Perspectives. Vol. 1. New York, NY: Psychology Press; 2002. pp. 219–244. [Google Scholar]
- 22.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 23.Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. New York, NY: Psychology Press; 2001. pp. 119–154. [Google Scholar]
- 24.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quirk GJ, Paré D, Richardson R, et al. Erasing fear memories with extinction training. J Neurosci. 2010;30(45):14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konorski J. Conditioned Reflexes and Neuronal Organization. London, England: Cambridge University Press; 1948. [Google Scholar]
- 27.Konorski J. Integrative Activity of the Brain. Chicago, IL: University of Chicago Press; 1967. [Google Scholar]
- 28.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 29.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 30.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberini CM, Ledoux JE. Memory reconsolidation. Curr Biol. 2013;23(17):R746–R750. doi: 10.1016/j.cub.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 32.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47(6):795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17(13):1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- 34.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase–mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21(1):283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 35.Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28(33):8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1(3):216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213(2):201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 38.Font L, Cunningham CL. Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2012;101(2):222–230. doi: 10.1016/j.pbb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17(6):306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 41.Saladin ME, Gray KM, McRae-Clark AL, et al. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 2013;226(4):721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94(1):30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18(6):357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 44.Soeter M, Kindt M. Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience. Front Behav Neurosci. 2015;9:122. doi: 10.3389/fnbeh.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biol Psychol. 2013;92(1):43–50. doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiol Learn Mem. 2009;91(1):23–31. doi: 10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue Y-X, Luo Y-X, Wu P, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336(6078):241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 51.Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- 52.Diener E, Emmons RA. The independence of positive and negative affect. J Pers Soc Psychol. 1984;47(5):1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- 53.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 54.Heidbreder CA, Hagan JJ. Novel pharmacotherapeutic approaches for the treatment of drug addiction and craving. Curr Opin Pharmacol. 2005;5(1):107–118. doi: 10.1016/j.coph.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Smoking cessation for weight-concerned women: group vs. individually tailored, dietary, and weight-control follow-up sessions. Addict Behav. 2006;31(1):115–127. doi: 10.1016/j.addbeh.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 56.Sartor GC, Aston-Jones G. Post-retrieval extinction attenuates cocaine memories. Neuropsychopharmacology. 2014;39(5):1059–1065. doi: 10.1038/npp.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma X, Zhang JJ, Yu LC. Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval-extinction interval. Psychopharmacology (Berl) 2012;221(1):19–26. doi: 10.1007/s00213-011-2545-4. [DOI] [PubMed] [Google Scholar]
- 58.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao-Ruiz P, Rotaru DC, van der Loo RJ, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14(10):1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 60.Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci U S A. 2013;110(50):20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agren T, Engman J, Frick A, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337(6101):1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 62.Oyarzún JP, Lopez-Barroso D, Fuentemilla L, et al. Updating fearful memories with extinction training during reconsolidation: a human study using auditory aversive stimuli. PLoS One. 2012;7(6):e38849. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golkar A, Bellander M, Olsson A, Ohman A. Are fear memories erasable?—reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Front Behav Neurosci. 2012;6:80. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17(10):512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millan EZ, Milligan-Saville J, McNally GP. Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol Learn Mem. 2013;101:26–32. doi: 10.1016/j.nlm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Auber A, Muthu Karuppasamy NS, Pedercini M, Bertoglio D, Tedesco V, Chiamulera C. The effect of postretrieval extinction of nicotine pavlovian memories in rats trained to self-administer nicotine. Nicotine Tob Res. 2014;16(12):1599–1605. doi: 10.1093/ntr/ntu110. [DOI] [PubMed] [Google Scholar]
- 67.Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology (Berl) 2013;226(4):631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asthana MK, Brunhuber B, Mühlberger A, Reif A, Schneider S, Herrmann MJ. Preventing the return of fear using reconsolidation update mechanisms depends on the met-allele of the brain derived neurotrophic factor val66met polymorphism [published online December 30, 2015] Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37(5):1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. J Fam Pract. 1998;46(6):460–464. [PubMed] [Google Scholar]
- 71.Shiban Y, Brütting J, Pauli P, Mühlberger A. Fear reactivation prior to exposure therapy: does it facilitate the effects of VR exposure in a randomized clinical sample? J Behav Ther Exp Psychiatry. 2015;46:133–140. doi: 10.1016/j.jbtep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 73.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


