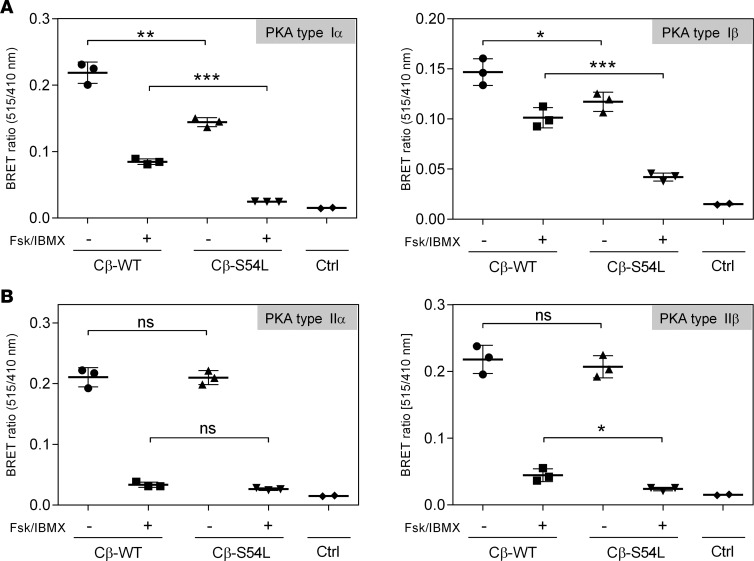
Figure 2. Mutant type I PKA holoenzyme stability is decreased in HEK293 cells.
Bioluminescence resonance energy transfer (BRET) experiments were used to test PKA type I (A) and type II (B) holoenzyme formation and dissociation. HEK293 cells were cotransfected with RLuc-tagged regulatory subunits (RIα or RIβ in panel A and RIIα or RIIβ in panel B) as a donor (emission 410 nm) and GFP-tagged catalytic subunits (wild-type [WT] or mutant [S54L] Cβ isoform 1) as an acceptor (emission 515 nm). Cells were treated either with buffer (basal condition, black boxes) or with 50 μM adenylyl cyclase activator forskolin (Fsk) and 100 μM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) to induce holoenzyme dissociation (stimulated condition, white boxes). Transfection of the empty RLuc8 vector was used as control (Ctrl). (A) The BRET ratio is significantly lower in basal and stimulated conditions for type I holoenzymes with the S54L mutant compared with the WT, indicating a decrease in mutant holoenzyme formation and stability. (B) No significant differences in BRET ratio were observed under basal conditions for the type II holoenzymes, indicating that the S54L mutation does not disturb the holoenzyme formed with type II regulatory subunits. Data from 3–6 replicates from 3 independent experiments are represented as dot plots and analyzed by unpaired t test with Sidak’s post hoc test for multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.