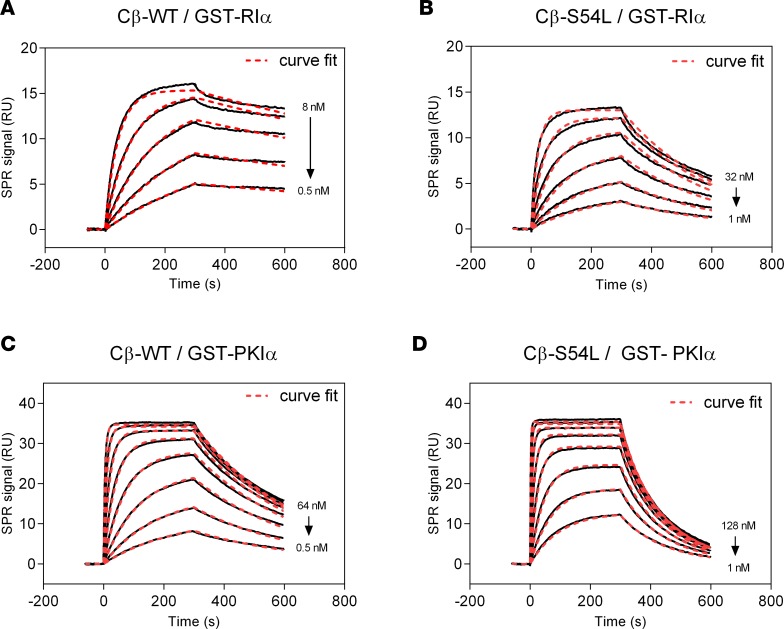
Figure 3. Binding of PKA-Cβ to physiological pseudosubstrate inhibitors.
Binding affinity of Cβ-WT (A and C) or Cβ-S54L (B and D) was determined using surface plasmon resonance (SPR). For this, recombinant RIα subunit (A and B) or the protein kinase inhibitor α (PKIα) (C and D) were captured on a sensor chip. Recombinant Cβ-WT (A and C) or Cβ-S54L (B and D) were injected for 300 seconds at different concentrations (association). Dissociation of the complex was monitored for 300 seconds. Equilibrium binding constants and rate constants were determined using a Langmuir 1:1 binding model (curve fits are depicted as red dashed lines in representative plots) and are summarized in Tables 1 and 2. The reduced affinities for RIα and PKIα determined for the mutant Cβ-S54L compared with the WT protein are mainly due to faster complex dissociation (see Tables 1 and 2 for dissociation rates). RU, response units.