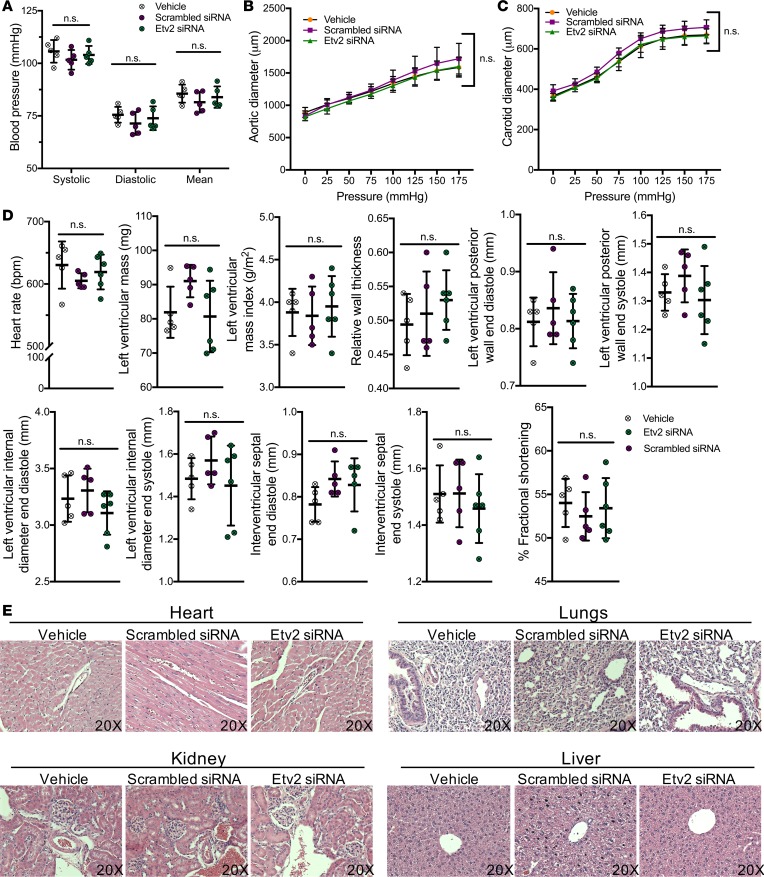
Figure 7. Etv2 siRNA peptide nanoparticle treatment do not adversely affect cardiovascular system and function.
Wild-type mice were intravenously treated with vehicle, scrambled siRNA, or Etv2 siRNA nanoparticles for 5 times, every other day. (A) Systolic, diastolic, and mean blood pressure measurements in control (vehicle and scrambled siRNA nanoparticles) or Etv2 siRNA nanoparticle–treated mice (n = 5/group). (B and C) Pressure diameter measurements of the (B) ascending aorta and (C) carotid artery over a range of pressures from 0 to 175 mmHg (n = 5/group). (D) Heart rate, left ventricular mass, left ventricular mas index, relative wall thickness, left ventricular posterior wall end diastole, left ventricular posterior wall end systole, left ventricular internal diameter end diastole, left ventricular internal diameter end systole, intraventricular septal end diastole, intraventricular septal end systole, and percentage fractional shortening in control (vehicle and scrambled siRNA nanoparticles) or Etv2 siRNA nanoparticle–treated mice, measured by echocardiogram analysis (n = 5/group). (E) Representative images of H&E-stained sections of heart, lungs, kidney, and liver harvested from control (vehicle and scrambled siRNA nanoparticles) or Etv2 siRNA nanoparticle–treated mice (original magnification, ×20). Data are presented as mean with standard deviation for all measurements. Statistical significance was analyzed by either 1-way ANOVA with Bonferroni’s multiple-comparison test (A and D) or 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (B and C).