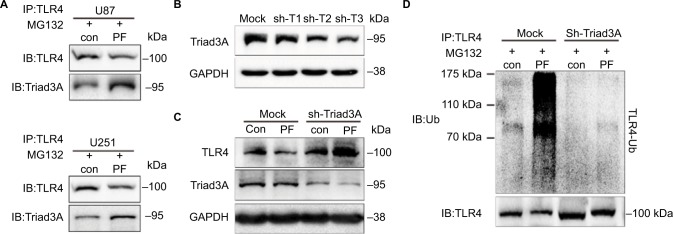
Figure 4.
Paeoniflorin enhances the interaction of Triad3A and TLR4, while sh-Triad3A disturbs paeoniflorin-induced degradation of TLR4 in glioblastoma cells.
Notes: (A) Detection of Triad3A binding with TLR4 was assessed by the immunoprecipitation of TLR4 in U87 and U251 cells treated with MG-132 (10 μM) for 6 h followed by paeoniflorin (20 μm) for 18 h. After incubation, whole cell lysates were immunoprecipitated using anti-TLR4 antibodies. Protein levels of Triad3A and TLR4 were measured by Western blotting. (B) U87 cells were transfected with 3 types of sh-Triad3A plasmids (sh-T1, sh-T2, and sh-T3) for 24 h and the expression of Triad3A was detected by Western blotting of whole cell lysates. (C) U87 cells transfected with mock or sh-Triad3A were incubated with paeoniflorin (20 μM) for 24 h followed by Western blotting of whole cell lysates to detect the expression of TLR4 and Triad3A. GAPDH was used as an internal control. (D) U87 cells transfected with mock or sh-Triad3A were treated with MG-132 (10 μM) for 6 h followed by with or without paeoniflorin (20 μm) for 18 h. Ubiquitination level of TLR4 were detected by the immunoprecipitation assay. All tests were performed in triplicate.
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TLR4, Toll-like receptor 4; Triad3A-shRNA, sh-Triad3A; IP,immunoprecipitatio; PF, paeoniflorin.