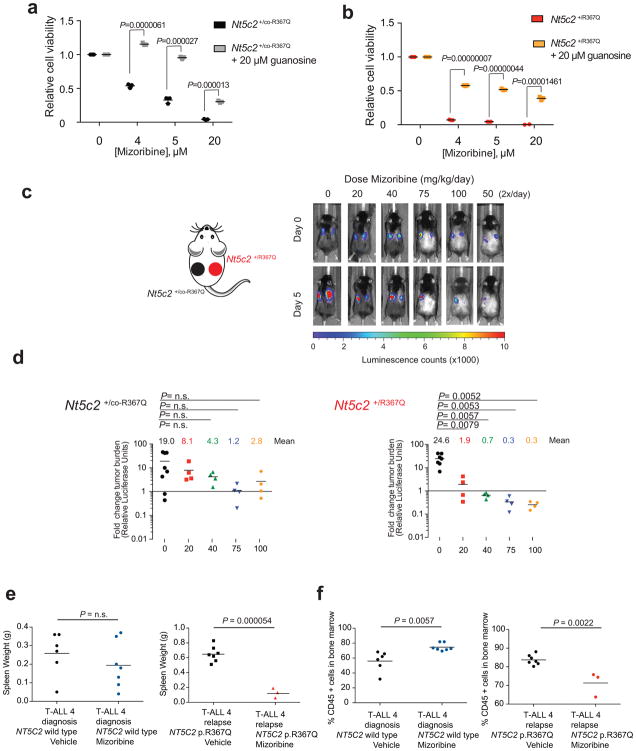
Extended Data Figure 6. Guanosine rescue of mizoribine sensitivity in vitro and mizoribine activity against NT5C2 p.R367Q mutant cells in vivo.
a, Cell viability assay showing drug responses of Nt5c2+/co-R367Q wild type primary mouse T-ALL cells to increasing doses of mizoribine in the presence of 20 μM guanosine (n=3 biological replicates). b, Cell viability assay results as in c documenting the effects of 20 μM guanosine in the response of isogenic Nt5c2+/R367Q mutant mouse T-ALL lymphoblasts to mizoribine (n=3 biological replicates). c, Analysis of tumor burden assessed by bioimaging in mice transplanted with Nt5c2+/co-R367Q wild type leukemia cells (left) or Nt5c2+/R367Q mutant leukemia cells (right) treated with a range of mizoribine doses (n = 8 mice for the vehicle group and n=4 mice per treated group). d, Quantification of data in c. e, Analysis of tumor burden assessed by spleen weight in mice allografted with NT5C2 wild type ALL-4 diagnosis or NT5C2 p. R367Q ALL-4 relapse patient derived leukemia cells treated with 100 mg kg−1 mizoribine b.i.d. (n=6 for diagnosis vehicle group, n=3 for relapse treated group and n=7 for diagnosis treated and relapse vehicle groups) f, Analysis of tumor burden assessed by % CD45+ cells in the bone marrow of mice allografted with NT5C2 wild type ALL-4 diagnosis or NT5C2 p.R367Q ALL-4 relapse patient derived leukemia cells treated with 100 mg kg−1 mizoribine b.i.d. (n=3–7 per group). Horizontal bar in a, b, d, e, and f indicates mean values. P values were calculated using two-tailed Student’s t-test.