Figure 3.
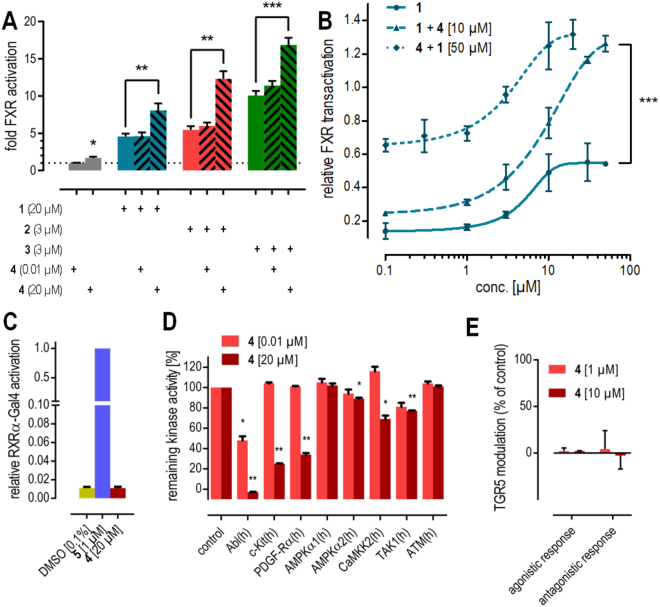
In vitro profiling of FXR modulation by 4. (A) At 20 µM, 4 significantly enhances transactivation efficacy of FXR agonists 1–3 in a full-length FXR reporter gene assay. (B) Dose-response curves of FXR agonist 1 and compound 4 in presence of a fixed concentration of the respective other agent confirms increased maximum relative transactivation without marked changes in EC50-values. The maximum relative transactivation exceeds the sum of the single compounds’ maximum relative transactivation suggesting synergy. (C) 4 does not activate FXR’s heterodimer partner RXR. (D) 4 is a potent inhibitor of the kinases Abl, c-Kit and PDGF-Rα but does not markedly inhibit kinases that interact with FXR. (E) 4 does not modulate the membrane bile acid receptor TGR5. *p < 0.05, **p < 0.01, ***p < 0.001.