Figure 4.
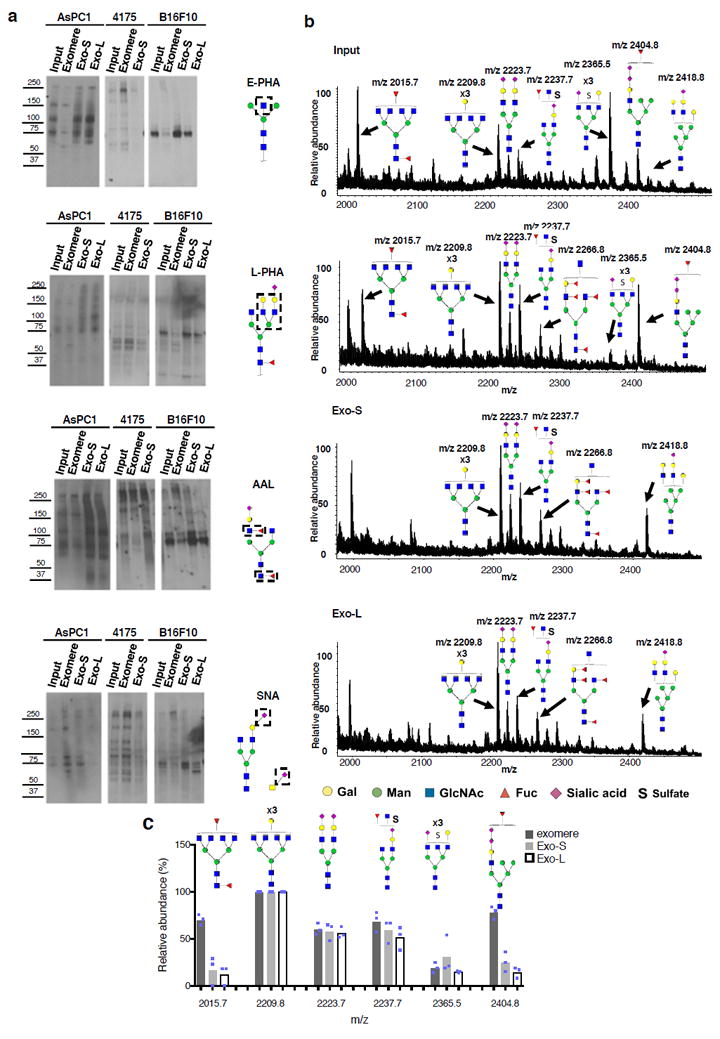
Characterization of N-glycosylation of proteins associated with exomere, Exo-S and Exo-L. (a) Lectin blotting analysis of N-glycan profile of proteins associated with exomeres versus exosome subpopulations Exo-S and Exo-L. Phaseolus vulgaris erythroagglutinin (E-PHA) and Phaseolus vulgaris leucoagglutinin (L-PHA) recognize bisected and branched N-glycans, respectively. Aleuria aurantia lectin (AAL) recognizes Fucα6GlcNAc and Fucα3GlcNAc. Sambucus nigra lectin (SNA) recognizes α-2,6-linked sialic acid. All experiments were repeated independently twice with similar results except for AAL and E-PHA blotting for B16-F10 and 4175 which were done once. (b) Mass spectrometric analysis of N-glycans of glycoproteins present in exomeres, Exo-S and Exo–L subsets of B16F10. One representative experiment of two biologically independent replicates is shown. (c) Comparison of the relative abundance of the top six most abundant N-glycan structures among exomere, Exo-S and Exo-L of B16F10. The assignments (m/z) [charge; neutral exchange] for MALDI-MS and nanoLC-ESI-MS/MS are the following: (2015.8 [-H; 0]; 1007.4a [-2H; 0]), (2209.8 [-H; 0]; 1104.4a [-2H; 0]), (2237.7b [-H; Na-H]; 732.57a [-3H; 0]), (2365.5b [-H;4K-4H]; 783.9a [-3H; 0] and 1182.4a,b [-2H; 4K-4H]), and (2404.8b [-H; 2K-2H]; 1201.9b [-2H; 2K-2H]). Data shown were quantified and normalized to the most abundant structure in the sample. Results are represented as average of three independent analytical measurements of one representative experiment. Statistical source data are provided in Supplementary Table 8, and unprocessed blots are provided in Supplementary Figure 7. Note: aThe product ion spectra for this species did not allow a complete structural assignment. bAssignments admit neutral exchanges of protons with cations in sialoglycans, including the presence of potassium and sodium.
