Introduction
Membranous nephropathy (MN) is one of the leading causes of nephrotic syndrome in adults. Some cases are associated with malignancy, infections, autoimmune systemic diseases, or drugs, but most are of an autoimmune nature, being referred to as primary (PMN).1 The natural course is variable and unpredictable: approximately one-third of patients with PMN undergo spontaneous remission,2 whereas most patients with persisting nephrotic syndrome will progress to end-stage renal disease within 10 years.3 A giant leap in understanding the pathophysiological mechanisms of these idiopathic cases was made in 2009 with the identification of the first target auto-antigen in the adult, the M-type phospholipase A2 receptor (PLA2R), a podocyte membrane glycoprotein.4 Circulating PLA2R-antibody (PLA2R-Ab) is detected in 70% to 80% of patients with PMN, whereas antibody to the subsequently identified podocyte antigen THSD7A is found in only 2% to 3% of patients.5
The care of patients with PMN has dramatically changed with the development of specific assays of circulating antibodies. Proteinuria was previously the only marker of disease activity, and immunosuppressive treatment indications and adjustments were essentially empirical. Although PLA2R-Ab is a specific PMN diagnostic biomarker, it also has an important prognostic value. Many studies reported that high titers of PLA2R-Ab are correlated with a lower risk of spontaneous or immunosuppressant-induced remission, a higher risk of nephrotic syndrome, and end-stage renal disease.6, 7, 8, 9, 10 Conversely, patients with low PLA2R-Ab levels have a higher probability of remission and achieve remission of proteinuria earlier than patients with high PLA2R-Ab levels.6, 7, 8, 9, 10 Even more, the time course of PLA2R-Ab under treatment is tightly correlated with clinical outcome, with a decrease of PLA2R-Ab predicting clinical remission, and an increase predicting a relapse.11 Whether complete depletion of PLA2R-Ab must be achieved to obtain remission remains a matter of debate.
Rituximab has been used since 2002 as treatment of PMN.12, 13, 14 Recent publications showed that rituximab induced PLA2R-Ab depletion and that reduction of PLA2R-Ab titer preceded remission of proteinuria by several months.10, 11, 15 The first B-cell–driven study asking the question of rituximab dosage showed that 1 or 2 infusions of rituximab 375 mg/m2 were comparable to the protocol using 4 weekly infusions and could dramatically reduce costs.16 More recent works focusing on PLA2R-Ab reduction rather than B-cell depletion suggested that higher doses of rituximab were needed.10, 11 However, the total dose to achieve complete remission remains uncertain and may vary from one patient to another.
We report here the case of a patient with a refractory nephrotic syndrome who was treated with a progressive increase in immunosuppressive drugs for 3 years without success until PLA2R-Ab disappeared, and only then a complete and sustained clinical remission occurred.
Case Presentation
A 57-year-old man developed ankle edema in September 2009. This led to the discovery of a nephrotic syndrome (laboratory results are summarized in Table 1) without renal failure or hematuria in September 2010. A renal biopsy was performed and revealed a stage-2 MN. On immunofluorescence, the parietal deposits were granular and stained for IgG, C3, and lambda and kappa light chains in the same proportion. Detection of PLA2R antigen in immune deposits was positive. Anti-proteinuric treatment with ramipril and furosemide, and oral anti–vitamin K anticoagulant were started.
Table 1.
Laboratory results
| Sep 2010 | Jan 2011 | Jun 2011 | Jan 2012 | April 2012 | Jun 2012 | Nov 2012 | May 2013 | Jun 2014 | Dec 2014 | Dec 2016 | May 2017 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine, μmol/l | 100 | 110 | 142 | 186 | 153 | 154 | 230 | 230 | 240 | 185 | 160 | 166 |
| Proteinuria, g/d | 4 | 15 | 20 | 12 | 8.5 | 6.3 | 14.5 | 3.2 | 1.5 | 0.6 | 0.25 | 0.15 |
| Albumin level, g/dl | 1.5 | 1.5 | 19 | 1.9 | 1.9 | 2.5 | 2.2 | 31 | 3.7 | 3.8 | 3.9 | 4.0 |
| PLA2R-Ab, ELISA | NA | NA | NA | 378 | 11 | 33 | 46 | 81 | 55 | 0 | 0 | 0 |
| PLA2R-Ab, IIFT | NA | 1/100 | 1/1000 | 1/500 | 1/50 | 1/100 | 1/100 | 1/500 | 1/500 | 0 | 0 | 0 |
| Treatment | RTX | RTX | Cyclo | Cyclo | Cyclo RTX |
cyclo, cyclosporine; ELISA, enzyme-linked immunosorbent assay; IIFT, indirect immunofluorescence test; NA, not available; PLA2R-Ab, phospholipase A2 receptor antibody; RTX, rituximab.
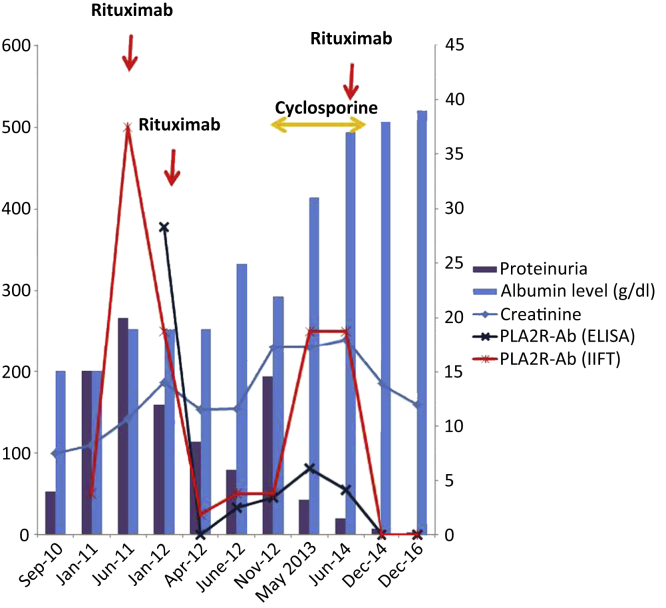
In June 2011, the patient still had nephrotic syndrome with rise in serum creatinine level to 142 μmol/l. He received a first line of immunosuppressive treatment with 2 infusions of rituximab 375 mg/m2 per week.
In January 2012, the patient still presented nephrotic syndrome with worsening of renal failure. He was then referred to our nephrology unit. Circulating PLA2R-Ab assessed by indirect immunofluorescence test (IIFT) and enzyme-linked immunosorbent assay (ELISA) (both EUROIMMUN, Lübeck, Germany), were at 1/500 and 378 RU/ml, respectively. Given the high titer of antibodies, the renal failure and the lack of complete CD19 lymphocyte depletion (CD 19 lymphocytes, 22/μl), another course of rituximab 375 mg/m2 weekly for 4 weeks was performed. Three months later, PLA2R-Ab decreased to 1/50 by IIFT and became undetectable (11 RU/ml) by ELISA, and in June 2012, the nephrotic syndrome had improved but PLA2R-Ab had raised to 1/100 and 33 RU/ml, respectively.
In November 2012, the patient presented a worsening of nephrotic syndrome with an increase of renal impairment, PLA2R-Ab level was 1/100 and 46 RU/ml. We subsequently started a second line of immunosuppressive treatment with cyclosporine 5 mg/kg per day. The patient achieved partial remission of nephrotic syndrome.
The patient was maintained on cyclosporine for 18 months and nephrotic syndrome improved dramatically. Soon after, a third line of immunosuppressive therapy was discussed because of the altered renal function, the high blood pressure, and persistent detectable PLA2R-Ab (respectively 1/500 dilution and 55 RU/ml). The patient refused a treatment with steroids and cyclophosphamide because of his propensity to develop diabetes mellitus. We performed a third course of rituximab treatment (375 mg/m2 weekly for 4 weeks). After the third infusion, PLA2R-Ab became undetectable and cyclosporine was withdrawn. Six months later, the patient achieved complete remission of nephrotic syndrome. Blood pressure was better controlled, allowing withdrawal of 2 antihypertensive medications. Two years later, nephrotic syndrome is still in complete remission, renal function is stable, and PLA2R-Abs are still undetectable by both techniques (Figure 1).
Figure 1.
Evolution of creatinine, serum albumin, proteinuria, and PLA2R-Ab. Creatinine is expressed in μmol/l, proteinuria is expressed by g/d, PLA2R-Ab is assessed by indirect immunofluorescence test and indirect enzyme-linked immunosorbent assay and expressed by RU/ml (left axis), and serum albumin is expressed by g/l (right axis).
Discussion
Our case first shows that immunological remission must be the ultimate goal in treating patients with PLA2R-Ab–positive PMN (Table 2). Complete clinical remission occurred only after complete immunological remission as defined by total disappearance of antibodies both by IIFT and ELISA. This result required not fewer than 4 courses of therapy, using 2 different immunosuppressive agents: rituximab, 2 doses; followed by rituximab, 4 doses; then cyclosporine; and finally rituximab, 4 doses. Although there is no information on the threshold level of antibody that should be reached, Bech et al.17 showed that persistence of PLA2R-Ab at the end of immunosuppressive treatment was associated with a high risk of relapse. A recent review proposed a serological-based approach to manage patients with MN. The authors suggest considering immunosuppressive therapy withdrawal if PLA2R-Ab level is reduced by 90% after 6 months.18 Our case suggests that complete PLA2R-Ab depletion rather than 90% PLA2R-Ab level reduction may predict clinical response and thus should be the ultimate goal of immunosuppressive therapy.
Table 2.
Distinct teaching points
| PLA2R-Ab levels should drive the lines of treatment |
| We should consider using a combination of therapies in more severe cases |
| Rituximab treatment is an efficacy therapy for MN but in severe or refractory cases calcineurin inhibitor could be given to limit proteinuria before rituximab administration. |
| PLAR-Ab level should be considered in the definition of remission, particularly in patients achieving only partial remission |
| The ultimate goal of the immunosuppressive treatment in PLA2R-Ab–positive patients with PMN should be complete disappearance of antibody by IIFT |
IIFT, indirect immunofluorescence test; MN, membranous nephropathy; PLA2R-Ab, phospholipase A2 receptor antibody; PMN, primary MN.
The second important information is the greater sensitivity of IIFT as compared with ELISA (Table 2). This was known at diagnosis19 but not reported during follow-up. In April 2012, while the patient was still nephrotic after the second course of rituximab (4 doses), ELISA was negative (11 RU/ml) but IIFT was still positive 1/50. This discrepancy may be partly explained by the high cutoff recommended by the manufacturer (14 RU/ml), but operationally, one should conclude that the definition of immunological remission requires both a negative IIFT and a negative ELISA, as nicely illustrated by this patient.
Third, this case leads to discussion of the optimal dose of rituximab in highly nephrotic patients who are losing a great deal of Ig (including the anti-CD20 antibody) in the urine, as well as the rationale for successive immunosuppressive therapies. Actually, the pharmacokinetics of rituximab and consequently the optimal dosing are not well established in nephrotic patients with PMN.10, 12, 13, 14 The doses used for the first time in 8 patients by Remuzzi’s group12 were 4 weekly infusions of rituximab (375 mg/m2), in analogy with standard treatment for B-cell lymphoma. A following study showed that a B-cell–driven approach with only 1 or 2 infusions of rituximab of 375 mg/m2 per week could allow reducing cost in comparison with the standard protocol of 4 weekly infusions.14 However, other trials continued to use the 4 doses regimen.13 Our recent randomized clinical trial (GEMRITUX) has shown the efficacy of rituximab (375 mg/m2 per week for 2 weeks) compared with standard anti-proteinuric treatment alone in achieving remission in patients with PMN.10 Rituximab decreased median PLA2R-Ab titer as early as month 3, but complete immunologic remission was induced in only 56% and 50% of the patients at months 3 and 6, respectively, which suggested that the dose of rituximab used might be too low. In the present case, the first course of treatment with 2 infusions of rituximab (375 mg/m2 day 1 and day 8) did not achieve CD19 depletion and was clinically inefficient; the second course of treatment with higher doses of rituximab (375 mg/m2 per week for 4 weeks) was given 6 months later. A third course of therapy based on cyclosporine (5 mg/kg per day) induced partial remission of nephrotic syndrome but worsened hypertension without immunological remission (IIFT stable 1/500, ELISA 81 then 55 RU/ml). A fourth course of treatment with rituximab (375 mg/m2 per week for 4 weeks) was then fully successful, inducing for the first time sustained, complete immunological and clinical remission. Of note, the same treatment regimen (rituximab 375 mg/m2 per week for 4 weeks), which induced complete remission after cyclosporine, was only partially efficient 18 months earlier. Our best hypothesis is that the first courses of rituximab were given in patients severely nephrotic, whereas the latter course was administered at a low level of proteinuria. This observation suggests that in highly nephrotic patients apparently unresponsive to rituximab, an initial short treatment with cyclosporine might allow response to rituximab via its hemodynamics and anti-proteinuric effect (Table 2).20, 21
Conclusion
This case suggests that the ultimate goal of the immunosuppressive treatment in PLA2R-Ab–positive patients with PMN should be complete disappearance of antibody by IIFT. PLA2R-Ab levels should drive the lines of treatment and one should consider using a combination of therapies in more severe cases. PLAR-Ab level should be considered in the definition of remission, particularly in patients achieving only partial remission.
Disclosure
All the authors declared no competing interests.
References
- 1.Ponticelli C., Glassock R.J. Glomerular diseases: membranousnephropathy–a modern view. Clin J Am Soc Nephrol. 2014;9:609–616. doi: 10.2215/CJN.04160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polanco N., Gutiérrez E., Covarsí A. Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schieppati A., Mosconi L., Perna A. Prognosis of untreated patients with primary membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 4.Beck L.H., Bonegio R.G.B., Lambeau G. M-type phospholipase A2 receptor as target antigen in primary membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofstra J.M., Beck L.H., Beck D.M. Anti-phospholipase A2 receptor antibodies correlate with clinical status in primary membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha E., Thiele I., Zahner G. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoxha E., Harendza S., Pinnschmidt H. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and nonnephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y.G., Choi Y.W., Kim S.Y. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol. 2015;42:250–257. doi: 10.1159/000440983. [DOI] [PubMed] [Google Scholar]
- 10.Dahan K., Debiec H., Plaisier E. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggenenti P., Debiec H., Ruggiero B. Antiphospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remuzzi G., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 13.Fervenza F.C., Cosio F.G., Erickson S.B. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 14.Cravedi P., Sghirlanzoni M.C., Marasà M. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]
- 15.Beck L.H., Jr., Fervenza F.C., Beck D.M. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cravedi P., Ruggenenti P., Sghirlanzoni M.C. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 17.Bech A.P., Hofstra J.M., Brenchley P.E. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9:1386–1392. doi: 10.2215/CJN.10471013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vriese A., Glassock R., Nath K. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dähnrich C., Komorowski L., Probst C. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Rojas-Rivera J., Fernández-Juárez G., Ortiz A. European multicentre and open-label controlled randomized trial to evaluate the efficacy of sequential treatment with tacrolimus-rituximab versus steroids plus cyclophosphamide in patients with primary membranous nephropathy: the STARMEN study. Clin Kidney J. 2015;8:503–510. doi: 10.1093/ckj/sfv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldman M., Beck L.H., Jr., Braun M. Membranous nephropathy: pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int Rep. 2016;1:73–84. doi: 10.1016/j.ekir.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]