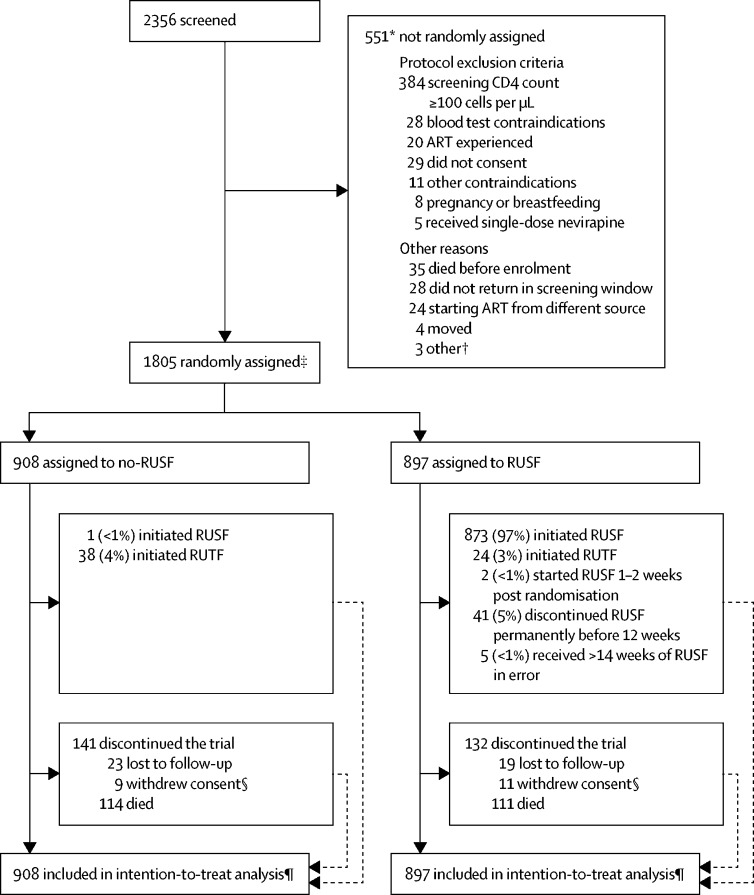
Figure 1.
Trial profile
ART=antiretroviral therapy. RUSF=ready-to-use supplementary food. RUTF=ready-to-use therapeutic food. *Reasons were not mutually exclusive, therefore total is more than the number of patients not randomly assigned treatment. †Considered too unwell (one patient), not able to comply with trial schedule (one patient), and no further details (one patient). ‡Ten patients ineligible after randomisation (four previously received ART, one RUSF contraindicated [milk allergy]), one 3 months pregnant, one had CD4 count ≥100 cells per μL at screening [38 cells per μL at enrolment], one randomly assigned 7 weeks after screening, two incorrect consent [one aged 14 years gave assent but without caregiver consent at enrolment; one aged 19 years old gave assent but caregiver consent was obtained on the basis of a self-reported age of 16 years at screening]). §Four patients assigned no-RUSF and two assigned RUSF were not formally lost to follow-up (they were seen in the clinic within 91 days of week 48). ¶Time-to-event analyses included all times at-risk from randomisation to the earliest of the event or last clinical follow-up if the event had not occurred (details on adherence to randomised strategy in appendix).