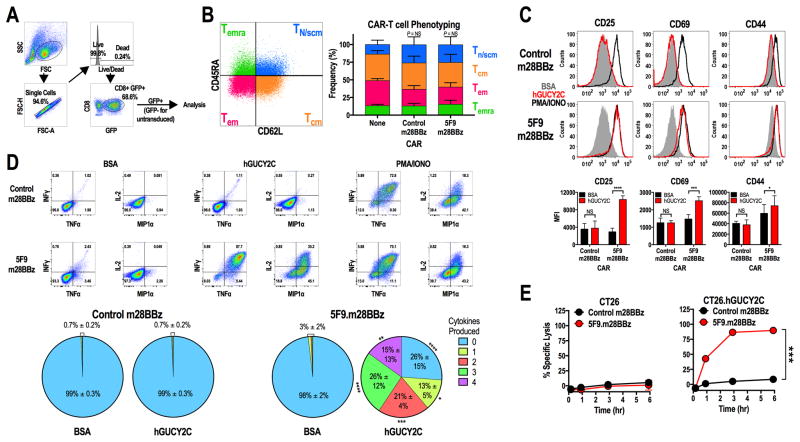
Figure 2. hGUCY2C-specific CARs mediate antigen-dependent T-cell activation and effector functions.
(A–E) Murine CD8+ T cells were left non-transduced (None) or transduced with control 1D3.m28BBz or 5F9.m28BBz CAR constructs as indicated. (A) Gating strategy for all analyses in B–D. (B) Representative CAR-T cell phenotyping plot based on CD45RA and CD62L. Two-way ANOVA; NS: not significant; Bars: mean ± SD from 2–3 independent experiments; Tn/scm: naïve or T memory stem cells; Tcm: central memory T cells; Tem: effector memory T cells; Temra: effector memory T cells expressing CD45RA. (C–D) 106 CAR-T cells were stimulated for 6 hours with plate-coated antigen (BSA or hGUCY2C) or PMA and ionomycin (PMA/IONO). T-cell activation markers (CD25, CD69, or CD44) and intracellular cytokine production (IFNγ, TNFα, IL2, and MIP1α) were then quantified by flow cytometry. Graphs indicate the mean ± SD (C) activation marker upregulation (MFI) and (D) polyfunctional cytokine production (% of CAR+ cells) from 3 independent experiments. (E) Parental CT26 or CT26.hGUCY2C mouse colorectal cancer cells in an E-Plate were treated with CAR-T cells (5:1 E:T ratio), media, or 10% Triton-X 100 (Triton), and the relative electrical impedance was quantified every 15 minutes for 10 hours to quantify cancer cell death (normalized to time=0). Percent specific lysis values were calculated using impedance values following the addition of media and Triton for normalization (0% and 100% specific lysis, respectively). Two-way ANOVA, B–E; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.