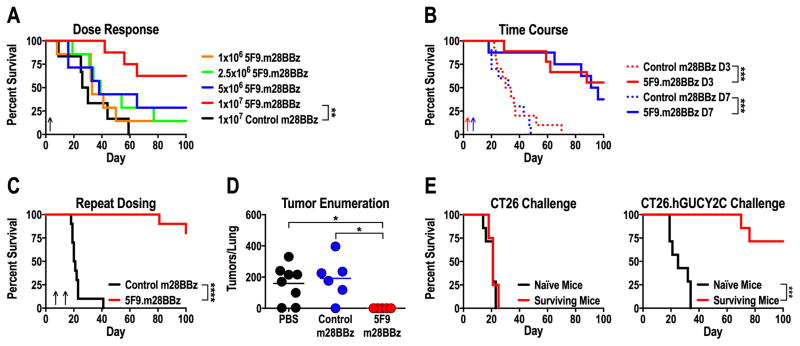
Figure 3. hGUCY2C CAR-T cells provide long-term protection in a syngeneic lung metastasis model.
(A–E) BALB/c mice were injected with 5x105 CT26.hGUCY2C cells via the tail vein to establish lung metastases. Control (4D5.m28BBz) or 5F9.m28BBz CAR constructs were transduced into murine CD8+ T cells. (A) Mice were treated 3 days later with 5 Gy total body irradiation (TBI) followed by 106–107 5F9.m28BBz (N=7–8/group) or 107 control (N=6) CAR-T cells. (B) Mice were treated on day 3 (D3) or day 7 (D7) with 5 Gy TBI followed by 107 control (N=10/group) or 5F9.m28BBz (N=9–10/group) CAR-T cells. (C) Mice were treated on day 7 with 5 Gy TBI followed by 107 control (N=10) or 5F9.m28BBz (N=12) CAR-T cells on day 7 and day 14. (D) Mice treated on day 7 with 5 Gy TBI and PBS or 107 control or 5F9.m28BBz CAR-T cells were sacrificed on day 18, lungs stained with India ink, and tumors/lung enumerated. One-way ANOVA; *p<0.05. (E) Surviving mice from B and C treated with 5F9.m28BBz CAR-T cells or naïve mice were challenged with 5x105 CT26 (N=4–7/group) or CT26.hGUCY2C (N=7/group) cells (re-challenge occurred 16–40 weeks after initial challenge). Log-rank Mantel-Cox test, A–C and E; **p<0.01, ***p<0.001, ****p<0.0001. Up arrows indicate CAR-T cell treatment days. Each panel indicates an independent experiment.