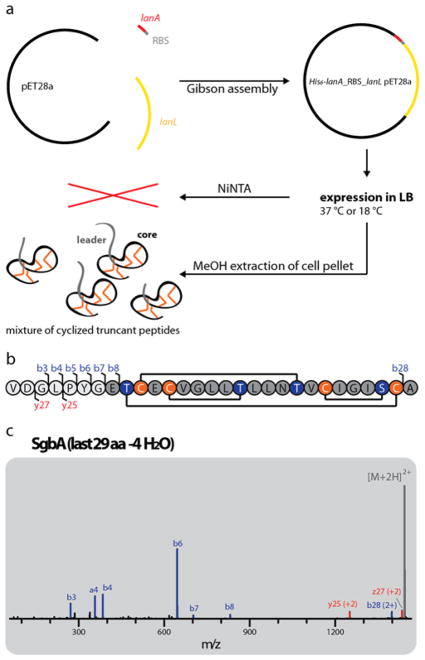
Figure 3.
(a) Schematic representation of the heterologous E. coli production system for the B2293 lanthipeptide globisporin. (b) Amino acid sequence of the last 29 residues of the SgbA precursor peptide showing the cyclization pattern known from venezuelin49 and streptocollin.56 Positions where b and y fragments were detected by tandem MS (including mass shifts due to water losses) are highlighted. (c) Tandem MS of the −4 H2O species of the extracted 29 amino acid (aa) peptide. The lack of detectable fragments in the region of Thr2-Cys21 (and overall low intensity of fragment ions) suggests formation of a methyllanthionine ring between these residues. For calculated and observed m/z values see Supporting Information Table S3. The tandem MS data only provides evidence of an overlapping ring topology that spans Dhb2 to Cys21. The topology the rings shown in (b) was predicted based on the high similarity of the globisporin system with the sequences producing venezuelin and streptocollin (Figure 2).