Abstract
Background
Casein glycomacropeptide is a peptide that lacks phenylalanine, tyrosine, and tryptophan. This profile may enable it to deplete phenylalanine, tyrosine, and tryptophan, and subsequently the synthesis of dopamine and serotonin in the brain. Dopamine- and serotonin-depleting amino acid mixtures have shown promise as acute antimanic treatments. In this study, we explore the depleting effects on amino acids, dopamine and serotonin as well as its actions on manic-like and other behavior in rats.
Methods
Casein glycomacropeptide and a selection of amino acid mixtures were administered orally at 2, 4, or 8 h or for 1 week chronically. Amino acid and monoamine levels were measured in plasma and brain and behavior was assessed in the amphetamine-hyperlocomotion, forced swim, prepulse inhibition, and elevated plus maze tests.
Results
Casein glycomacropeptide induced a time-dependent reduction in tyrosine, tryptophan, and phenylalanine in brain and plasma which was augmented by supplementing with leucine. Casein glycomacropeptide +leucine reduced dopamine in the frontal cortex and serotonin in the hippocampus, frontal cortex, and striatum after 2 and 4 h. Casein glycomacropeptide+leucine also had antimanic activity in the amphetamine-induced hyperlocomotion test at 2 h after a single acute treatment and after 1 week of chronic treatment.
Conclusions
Casein glycomacropeptide-based treatments and a branched-chain amino acid mixture affected total tissue levels of dopamine in the frontal cortex and striatum and serotonin in the frontal cortex, striatum, and hippocampus of rats in a time-dependent fashion and displayed antimanic efficacy in a behavioral assay of mania.
Keywords: casein glycomacropeptide, branched-chain amino acids, dopamine depletion, serotonin depletion, tyrosine depletion, tryptophan depletion, mania
Significance Statement
Casein glycomacropeptide (CGMP) is a peptide that lacks phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp), a profile that may enable it to deplete Phe, Tyr, and Trp, and subsequently the synthesis of dopamine (DA) and serotonin (5-HT) in the brain.
In the present work, a new dietary intervention paradigm in acute mania based on casein glycomacropeptide is proposed. The mechanism relies on rapid changes in brain and peripheral amino acid and monoaminergic levels in a time-dependent fashion. The observed changes correlate with antimanic efficacy in a behavioral assay of mania.
Introduction
Bipolar disorder is a serious condition with an estimated lifetime prevalence rate ranging from 2.8% to 6.5% (Bauer and Pfennig, 2005). Although the biological mechanism by which mania occurs is not yet known, the antimanic efficacy of dopaminergic D2 receptor antagonists as well as the dopaminergic resemblance of manic symptoms (e.g., experience of extreme and intense energy, a reduced need for sleep, increased talkativeness) suggest that an overactivity in dopamine (DA) neurotransmission may play a role. Recent in vivo imaging evidence also suggests a contributory role for DA in mania (Jauhar et al., 2017). Antipsychotics are currently the fastest acting drugs for treating mania, with a 30% reduction on a mania rating scale (YMRS) occurring within 7 days, and a 50% to 60% reduction after 4 weeks (Tohen et al., 2000). Antipsychotics have problematic and potentially serious side effects at therapeutic doses, such as extrapyramidal symptoms and metabolic disturbances (e.g., weight gain, hyperlipidemia, hyperglycemia).
Interestingly, 2 clinical studies have reported that the depletion of brain levels of the DA precursor amino acids (AAs), Phe and Tyr, by consuming an AA mixture that lacks Phe and Tyr (McTavish et al., 2001b) or a mixture of branched-chain amino acids (BCAAs), that is, leucine (Leu), isoleucine (Ile), and valine (Val) (Scarna et al., 2003), produces a rapid improvement in manic symptoms (within 6 hours) and has a persistent benefit for 1 week with daily treatment, although it should be noted that these patients were in many cases still receiving their usual antimanic and/or psychotic drug treatments, and the observed effects can therefore not be attributed solely to the AA-depleting mixtures.
The same depleting AA mixtures have also been shown to inhibit amphetamine-induced manic behavior and DA neurotransmission in humans (McTavish et al., 1999b, 2001b; Leyton et al., 2004) and rats (McTavish et al., 1999a, 2001a; Le Masurier et al., 2004, 2006; Jaskiw et al., 2006; Bongiovanni et al., 2008, 2012; Brodnik et al., 2013). Together, these preliminary observations suggest that the depletion of Tyr and Phe in the brain by dietary means could have potential as a nutritional supplementary treatment for acute mania.
The depletion of tryptophan (Trp) by consuming a Trp-free AA drink has also been shown to improve mania ratings (Applebaum et al., 2007), but although Trp depletion does not appear to affect mood in healthy subjects (Benkelfat et al., 1994), depressed patients in remission receiving selective serotonin reuptake inhibitors (Van der Does, 2001) or people with a family history of depression (Benkelfat et al., 1994; Klaassen et al., 1999) have been shown to experience depression symptoms during acute Trp depletion. A selective depletion of Tyr may therefore be desirable.
Free AA mixtures have a notoriously foul taste that hampers compliance and tolerability. Casein glycomacropeptide (CGMP) (e.g., Lacprodan CGMP-20, Arla Foods Ingredients Group P/S) is a protein isolated from whey during the cheese-making process. It lacks Phe, Tyr, and Trp and is rich in large neutral amino acids (LNAAs) such as threonine (Thr) and isoleucine (Ile) (see Table 1), which compete with Phe, Tyr, and Trp (and other large neutral amino acids or LLNAs) for entry at the same active AA transporter in the blood-brain barrier. CGMP is currently used as a protein supplement for phenylketonuria patients. However, its unique AA profile could be used to deplete brain levels of Phe, Tyr, and Trp. With the benefit of improved compliance due to better palatability and based on the promising, rapid effects observed with AA-based depletion strategies in manic patients (McTavish et al., 2001b; Scarna et al., 2003), CGMP could be useful as a dietary treatment for acute manic attacks. This has also been previously suggested (Badawy, 2013).
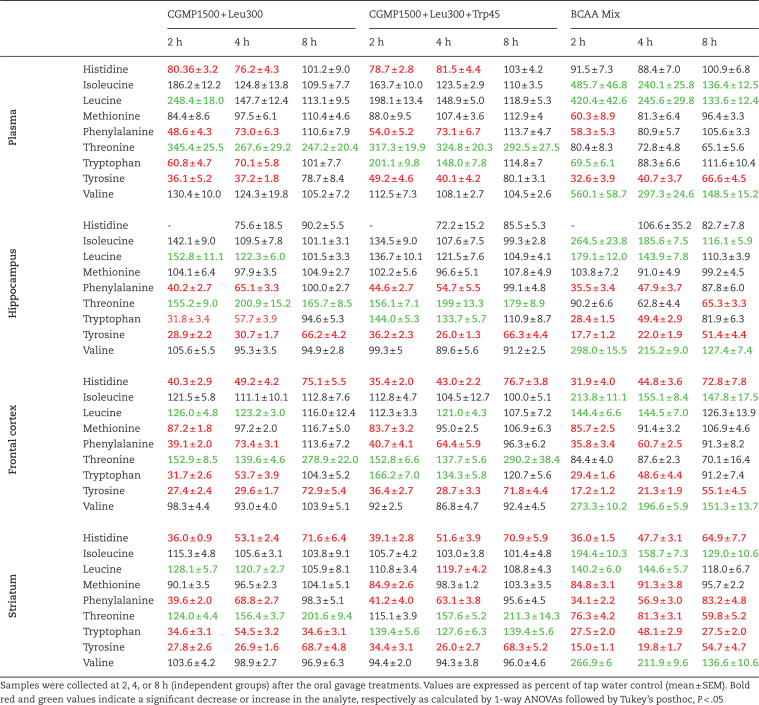
Table 1.
The Time-Dependent Effects on Tryptophan, Tyrosine, and Other Large Neutral Amino Acid Levels in Plasma, Hippocampus, Prefrontal Cortex, and Striatum by CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg), and BCAA Mixture (Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg)
In this study, we used rats to investigate (1) whether CGMP (with or without added Leu) depletes Phe, Tyr, and Trp in plasma and brain tissue and whether DA and/or 5-HT levels in the brain are affected; (2) the time dependency of any such effects; (3) whether CGMP supplemented with Trp can prevent depletion of 5-HT; and (4) whether CGMP has activity in behavioral tests for mania, anxiety, depression, and schizophrenia.
Methods And Materials
Animals
Male Sprague Dawley rats weighing approximately 300 g (7–8 weeks old) on the day of testing were obtained from Taconic and housed in pairs in acrylic cages (Cage 1291H Eurostandard Type III H, 425×266×185 mm, Techniplast) under a 12-hour light cycle (lights on from 05:00 to 17:00) in a temperature- (20±2ºC) and humidity-controlled (60±5%) environment. All animals were kept in the same room with the aforementioned conditions for at least 1 week before the start of each experiment. Each cage had bedding material made of wooden chips, and the animals had access to a wooden stick to bite, nesting material, and metallic tunnel shelter. The animal rooms and all experimental facilities were protected from outside noise. The behavioral procedures were carried out in specially equipped rooms in the animal facility between 8:00 and 10:00 pm. Food and water were available ad libitum. Twenty-four hours before sample collection or behavioral testing, rats were given a low protein diet (9% crude protein, Sniff) to minimize experimental variation due to differences in dietary protein intake. Different cohorts of rats were used for the different behavioral tests, so that each rat was exposed to only one test, except for locomotor activity that was measured prior to the forced swim test. All animal procedures were approved by The Danish National Committee for Ethics in Animal Experimentation (2012-15-2934-00254).
Treatments
CGMP (Lacprodan CGMP-20), Leu, Ile, Val, Trp, and whey protein (Lacprodan DI-9224) was supplied by Arla Foods Ingredients Group P/S and all AAs were the l-enantiomer. The treatments were dissolved in tap water, the pH neutralized and administered via a Teflon feeding tube (18G/75 mm, Agntho’s) in a volume of 10 mL/kg. For the amphetamine-induced hyperlocomotion (AIH) test, d-amphetamine hemisulfate salt (Sigma Aldrich) was dissolved in saline and administered i.p. at a dose of 1 mg/kg and a volume of 1 mL/kg. All solutions were prepared freshly each day.
Dose Selection
The doses selected for CGMP in this study were based on other rat depletion studies where AA mixtures lacking Phe and Tyr (McTavish et al., 1999a, 2001a; Le Masurier et al., 2004; Jaskiw et al., 2006; Bongiovanni et al., 2008, 2012; Brodnik et al., 2013) or BCAA mixtures (Le Masurier et al., 2006) were used. In these studies, total AA amounts of 1000 mg/kg were administered i.p. In this study, we opted for oral administration and a CGMP dose of 1500 mg/kg. CGMP formulations that could be effectively used in humans have been described in detail (Badawy, 2013). The doses chosen for the BCAAs (Le Masurier et al., 2006) and Trp (Le Masurier et al., 2006) were based on previous depletion studies in rats. Table 2 ahows the Amino Acid Composition of CGMP (Lacprodan CGMP-20) and Whey Protein (Lacprodan DI-9224) and the Amounts of Amino Acids Received by a 300-g Rat at a Dose of 1500 mg/kg. Table 3 shows the Amino Acid Doses Received by a 300-g Rat for Amino Acid-Depleting Treatments.
Table 2.
The AA Composition of CGMP (Lacprodan CGMP-20) and Whey Protein (Lacprodan DI-9224) and the Amounts of AAs Received by a 300-g Rat at a Dose of 1500 mg/kg
| CGMP | Whey Protein | |||
|---|---|---|---|---|
| Composition (%) | Dose for 300-g rat (mg) | Composition (%) | Dose for 300-g rat (mg) | |
| Alanine | 6.6 | 29.7 | 5.6 | 25.2 |
| Arginine | 0.3 | 1.4 | 2.2 | 9.9 |
| Apartate | 3.4 | 15.3 | 11.5 | 5.2 |
| Asparagine | 2.2 | 9.9 | ||
| Glutamate | 12.2 | 54.9 | 18.6 | 8.4 |
| Glutamine | 2.8 | 12.6 | ||
| Cysteine | 0.1 | 0.5 | 2.5 | 11.3 |
| Glycine | 1.2 | 5.4 | 1.7 | 7.7 |
| Histidine | 0.1 | 0.5 | 1.8 | 8.1 |
| Isoleucine | 11.3 | 50.9 | 7.1 | 32.0 |
| Leucine | 2.4 | 10.8 | 11.5 | 51.8 |
| Lysine | 6.6 | 29.7 | 10.3 | 46.4 |
| Methionine | 2.2 | 9.9 | 2.5 | 11.3 |
| Phenylalanine | 0.2 | 0.9 | 3.2 | 14.4 |
| Proline | 12.8 | 57.6 | 6.9 | 31.1 |
| Serine | 8.1 | 36.5 | 5.2 | 23.4 |
| Threonine | 18.3 | 82.4 | 7.7 | 34.7 |
| Tryptophan | 0.1 | 0.5 | 1.8 | 8.1 |
| Tyrosine | 0.0 | 0.1 | 3.1 | 14.0 |
| Valine | 9.1 | 41.0 | 6.3 | 28.4 |
For whey protein, the aspartate and asparagine values as well as the glutamate and glutamine values are combined.
Table 3.
Amino Acid Doses Received by a 300-g Rat for Amino Acid-Depleting Treatments
| Dose for a 300-g rat (mg) | |||
|---|---|---|---|
| CGMP1500+Leu300 | CGMP1500+ Leu300+Trp45 | BCAA Mix | |
| Alanine | 29.7 | 29.7 | - |
| Apartate | 15.3 | 15.3 | - |
| Arginine | 1.4 | 1.4 | - |
| Asparagine | 9.9 | 9.9 | - |
| Cysteine | 0.5 | 0.5 | - |
| Glutamate | 54.9 | 54.9 | - |
| Glutamine | 12.6 | 12.6 | - |
| Glycine | 5.4 | 5.4 | - |
| Histidine | 0.5 | 0.5 | - |
| Isoleucine | 50.9 | 50.9 | 135.0 |
| Leucine | 100.8 | 100.8 | 180.0 |
| Lysine | 29.7 | 29.7 | - |
| Methionine | 9.9 | 9.9 | - |
| Phenylalanine | 0.9 | 0.9 | - |
| Proline | 57.6 | 57.6 | - |
| Serine | 36.5 | 36.5 | - |
| Threonine | 82.4 | 82.4 | - |
| Tryptophan | 0.5 | 14.0 | - |
| Tyrosine | 0.1 | 0.1 | - |
| Valine | 41.0 | 41.0 | 135.0 |
| Total | 540.1 | 553.6 | 450.0 |
Sample Collection
The animals were beheaded with a sharp guillotine without anesthesia whereafter blood and brain samples were collected. Trunk blood was collected into K3-EDTA coated tubes (Terumo, Venosafe) standing in ice. Tubes were quickly inverted and centrifuged at 3400 x g for 10 min at 4°C. The resulting plasma fraction and brain tissues were stored at -80°C until analysis. Hippocampus, frontal cortex, and striatum regions were rapidly dissected on ice, snap-frozen with dry ice, and stored at -80°C until analysis. The hippocampus was dissected as the ventral and dorsal regions between anterior-posterior coordinates (AP) of approximately -1.92 mm and -6.84 mm from bregma (Paxinos and Watson, 2014). The frontal cortex was dissected as the cortex area between approximately AP=6.12 mm and 2.52 mm from bregma. The striatum was dissected as the dorsal (caudate putamen) and ventral (nucleus accumbens) regions between approximately AP=3.72 mm and 0.36 mm from bregma.
High Pressure Liquid Chromatography
Brain samples were mixed at a ratio of 1:5 (w/v) with 0.2 M perchloric acid and homogenized for 2×2 seconds with a probe sonicator (model UW2200; Bandelin Electronics) at 70% power. The samples were centrifuged at 4°C for 30 min at 21000 x g and the supernatant transferred to Costar cellulose acetate filter tubes (0.22 µm; Corning Inc) and centrifuged at 4°C for 10 min at 21000 x g.
For the measurement of DA, 5-HT, and norepinephrine (NE), the filtrate was injected directly into the high pressure liquid chromatography system. For the measurement of Tyr and Trp in the first experiment, 0.2 M perchloric acid was used to dilute the samples a further 80x. For the measurement of AAs in the second experiment, the samples were diluted a further 80x for Glu, Asp, Gln, Ser, Ala, Arg, Gly, His, and Met and 8x for the remaining AAs. Plasma samples were prepared by adding perchloric acid to yield a final concentration of 0.2 M and then underwent the same centrifugation and filtration process as described for the brain samples. Plasma samples were diluted 100x for the measurement of Tyr and Trp in Experiment 1 as well as for the AAs in Experiment 2.
Chromatographic conditions were as follows: (1) for the neurotransmitter measurements in Experiments 1 and 2: equipment consisted of Thermo Scientific Ultimate 3000 model isocratic pump and autosampler equipped with a Hypersi BDS C18 3 μm, 3×150 mm particle column kept at 28°C. Detection was carried out using a Thermo Scientific Dionex model 6011RS ultra Coulometric Analytical cell (E1: -150 mV: E2: +250 mV vs Pd reference). The column was maintained at 27 °C while eluting the analytes with a MDTM mobile phase (Thermo Scientific Dionex Test Phase, 70–3829) at a flow rate of 0.5 mL/min. (2) Tyr and Trp measurement in Experiment 1: the same conditions were used as in (1) but with cell potentials at E1: +250 mV: E2: +550 mV vs Pd reference. (3) AA profile in Experiment 2: equipment consisted of Thermo Scientific Ultimate 3000 model 4-line gradient pump, autosampler and fluorometric detector equipped with a Kinetex EVO C18 5 µm 4.6×150 mm particle column kept at 40°C. Mobile phase A consisted of 10 mM Na2HPO4 adjusted to pH 7.8 with phosphoric acid and filtered through a 0.2-µm membrane under vacuum. Mobile phase B consisted of 1:1 methanol to acetonitrile. The gradient was as follows: 5 min equilibration at 3% mobile phase B and gradually increased to 60% mobile phase B over 20 min followed by 100% mobile phase B for 3 minutes. The flow rate was 1 mL/min and detection was carried out at 337 nm (excitation) and 442 nm (emission). The samples and standards underwent an in-needle precolumn o-phthaldialdehyde derivatization reaction as previously described (Dionex Corporation, 2015). All mobile phase components, standards and reagents were purchased from Sigma Aldrich.
Behavioral Measurements
All behavioral tests were carried out at 3 to 4 h after the start of the dark phase. Testing in the dark phase was performed to ensure a consistently quiet testing environment throughout the study, and possibly a more natural behavior pattern due to rats being nocturnal. The rats were allowed at least 1 h to acclimatize to the testing rooms. Different cohorts of rats were used for different behavioral tests so that each rat was exposed to only one behavioral test, except for the locomotor activity test that was carried out before the forced swim test.
Amphetamine Induced Hyperlocomotion (AIH)
The AIH test is a widely used test for the detection of antimanic activity (Pereira et al., 2014). This test was developed based on the verification that amphetamine can induce mania-like symptoms in healthy individuals and mood stabilizers, as lithium and valproate, can reverse such amphetamine effects (Willson et al., 2005; Gould et al., 2007). Briefly, rats were placed in a square (1 x 1 m) arena with a video camera mounted above the arena. Baseline locomotor activity was recorded for 45 min, after which rats received an i.p. injection of amphetamine (1 mg/kg) and were placed back into the arena to record locomotor activity for a further 45 min. One group of rats received a saline injection (and tap water oral gavage) to demonstrate amphetamine-induced hyperlocomotion. The distance moved (cm) by each rat was measured using EthoVision XT video tracking software (version 12; Noldus Information Technology, Waacheningen, The Netherlands), and the data were collapsed into 5-min bins.
Pre-Pulse Inhibition (PPI)
The PPI test was carried out as previously described (Hougaard et al., 2011). Briefly, the PPI testing was carried out in 2 chambers (San Diego Instruments) with 70 dB(A) white background noise. Animal movement was transduced from the test tubes (∅ 8.2 cm) through a piezoelectric accelerometer. The test session consisted of 5 min acclimatization; 5 startle stimuli of 120 dB; 35 randomized pre-pulse, startle, and no stimuli (10 startle stimuli of 120 dB(A); 5 each of 4 different levels of pre-pulses plus startle stimulus [72,74, 78, and 86 dB(A), denoted PPI72, PPI74, PPI78, and PPI86], and 5 trials with background noise only) followed by 5 final startle stimuli of 120 dB(A). The individual stimuli were randomized between 10 and 20 s (mean 15 s). The average amplitude (AVG) of the startle response was calculated as the mean of the 5 initial (AVGinitial), 10 middle (AVGmiddle), and 5 final (AVGfinal) stimuli for each of the exposed groups. PPI was expressed as percent reduction in the averaged 5 AVGs for each PPI compared with the average of the 10 middle startle trials: %PPI=100 − [(AVG at pre-pulse+startle trial)/(AVG at startle trial)]×100%.
Elevated Plus Maze (EPM)
The EPM is widely used for the screening of anxiolitic- or anxiogenic-like effects of drugs. The test was carried out as previously described (Fischer et al., 2012). The maze was 80 cm above the ground with 2 opposite open arms and 2 closed arms measuring 50 cm×10 cm. Rats were placed in the center of the maze facing one of the closed arms. Circadian rhythm has been shown to affect rodent behavior in the EPM (Jones and King, 2001; Bertoglio and Carobrez, 2002), and care should therefore be exercised when directly comparing these EPM results with light-phase studies. The time spent by the rats in the open arms of the maze was measured during a 5-min test session using EthoVision XT video tracking software.
Forced Swim Test (FST)
The FST is a widely used test for screening of antidepressant-like activity (Slattery and Cryan, 2012). The animals were exposed to the FST as previously described (Liebenberg et al., 2015; Kirkedal et al., 2017). The animals were placed individually to swim in acrylic cylinders (24 cm diameter x 60 cm high containing 40 cm of water at 24±1ºC) for 15 min (pre-test). After 24 h the animals were exposed to a 5-min test session that was recorded and later analyzed by an independent scorer blinded to the treatment groups. After each trial, the water of the cylinders was exchanged.
Open Field Test (OFT)
The OFT is widely used for assessing locomotor activity and as a tool to identify false-positive effects in tests that are dependent on the locomotor activity such as the FST (Slattery and Cryan, 2012). Immediately before the FST, the rats were placed in an open field arena (1m x 1m with 40-cm-high walls) for 5 min as previously described (Liebenberg et al., 2010). The total distance moved (cm) was quantified using Noldus Ethovision XT12 (Noldus Information Technology, Waacheningen, The Netherlands) video tracking software.
Data Analysis and Statistics
For DA, 5-HT, NE, and AA measurements, 1-way ANOVAs (followed by Tukey’s posthoc tests where appropriate) were used. The AA analyses were not corrected for multiple comparisons, but the repeatable patterns across sample types and time points indicate that the changes were not due to chance effects. For the AIH and PPI tests, repeated measures 1-way ANOVAs were performed. For the EPM, FST, and OFT, 1-way ANOVAs followed by Tukey’s posthoc tests were used where appropriate. Statistics were calculated using SPSS (version 22, SPSS Inc). The ANOVA results for the behavioral tests are stated in the Results section, and the ANOVA results for amino acid and neurotransmitter analyses are given in Supplementary Tables 1 and 2.
Study Design
Experiment 1: Dose-Determination and the Effects of Supplementing CGMP with Leu
Rats were treated with a low (1500 mg/kg) or high (3000 mg/kg) dose of CGMP via a single oral gavage administration. Two hours later, plasma and brain samples (hippocampus and frontal cortex) were collected to investigate the depleting action on brain and plasma Tyr, Trp, and brain DA and 5-HT. To investigate the additive depleting effect of Leu, separate groups of rats were treated with CGMP (1500 mg/kg)+Leu (300 mg/kg) or CGMP (3000 mg/kg)+Leu (600 mg/kg). A BCAA mixture, Leu (600 mg/kg)+Ile (450 mg/kg)+Val (450 mg/kg), was used as a positive control and tap water as a negative control. A group treated with whey protein (1500 mg/kg) was included as a reference. Tap water controls had n=18 and the other treatments had n=8.
Experiment 2: Time Dependency, Effects Supplementing with Trp, and a More Complete AA Profile
Rats were treated with a single oral gavage with CGMP (1500 mg/kg)+Leu (300 mg/kg) at 2, 4, or 8 h before decapitation. Plasma and brain samples (hippocampus, frontal cortex, and striatum) were collected to measure DA, 5-HT, NE, histidine (His), Ile, Leu, methionine (Met), Phe, threonine (Thr), Trp, Tyr, and Val. To explore whether a selective Tyr/Phe/DA depletion can be attained by supplementing with Trp, a group of rats was treated with CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg) at the same time points. A BCAA mixture, Leu (600 mg/kg)+Ile (450 mg/kg)+Val (450 mg/kg), was used as a positive control and tap water as a negative control. Group sizes were n=8 to 10.
Experiment 3: Behavioral Effects of CGMP
The same treatments used in Experiment 2 were used in the Experiment 3 with the inclusion of a whey protein (1500 mg/kg) control. To investigate acute effects, rats were exposed to a behavioral test at 2 or 8 h after a single oral gavage treatment. To investigate the effects of repeated treatments, rats were exposed to a behavioral test after 1 week of daily administration and 8 h after the final treatment. The behavioral tests used were as follows: AIH (manic-like behavior), PPI (schizophrenia-like behavior), EPM (anxiety-like behavior) and FST (depression-like behavior). Group sizes were n=10 to 12.
RESULTS
Experiment 1: Dose Determination and the Effects of Supplementing CGMP with Leu
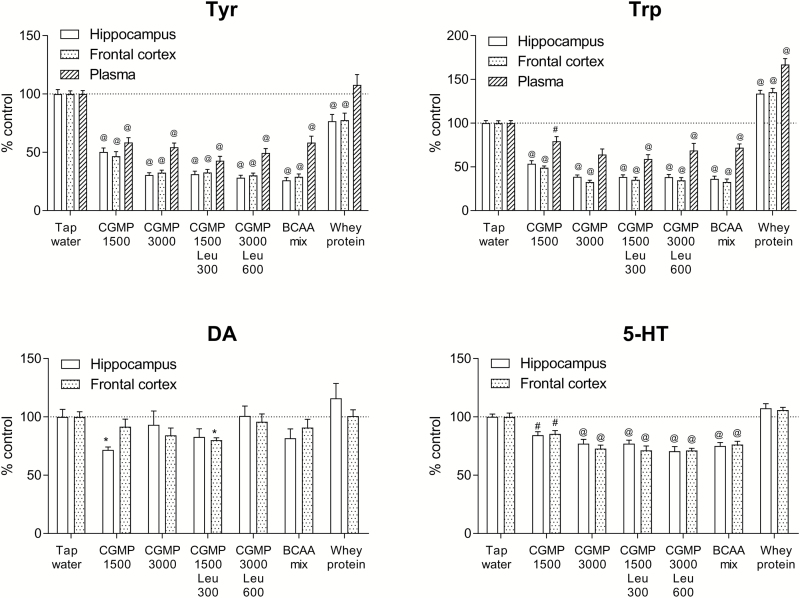
Both Tyr and Trp were significantly depleted (P<.001) by all treatments in the hippocampus, frontal cortex, and plasma, except for whey protein, which induced an increase in Trp in brain and plasma (P<.001) (Figure 1). DA levels were significantly reduced by CMGP 1500 mg/kg to 71.7% of control in the hippocampus and by CGMP 1500 mg/kg+Leu 300 mg/kg to 80.1% of control in the frontal cortex (P<.05) (Figure 1). The 5-HT levels in both the hippocampus and frontal cortex were depleted by the treatments (P<.01), except by whey protein (Figure 1). The CGMP (1500 mg/kg)+Leu (300 mg/kg) treatment was the most efficient treatment to reduce the levels of Tyr, Trp, DA, and 5-HT. The increase in Trp (to approximately 134% of control in brain and 167.1% of control in plasma) after whey protein treatment is likely explained by the relatively high content of Trp in whey compared with CGMP (see Table 2). The ANOVA results for Experiment 1 are summarized in Supplementary Table 1.
Figure 1.
The effects on Tyr and Trp in plasma and Tyr, Trp, DA, and 5-HT in the hippocampus and frontal cortex by tap water, CGMP (1500 mg/kg), CGMP (3000 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (3000 mg/kg)+Leu (600 mg/kg), BCAA mixture (i.e. Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg), or whey protein 2 h after oral gavage treatment. Values are expressed as percent of tap water control (mean±SEM). 1-way ANOVA followed by Tukey’s posthoc; *P<.05, #P<.01, @P<.001.
Experiment 2: Time Dependency, the Effects of Trp Supplementation, and a More Detailed AA Profile
CGMP (1500 mg/kg)+Leu (300 mg/kg) and the BCAA mixture depleted Tyr, Trp, and Phe after 2 h in plasma and brain, started to normalize after 4 h, and returned to control levels after 8 h, except for Tyr, which remained reduced in both plasma and brain (Table 1). By adding Trp (45 mg/kg) to CGMP+Leu, the depletion of Trp was prevented and induced a significant increase in Trp levels (in plasma and brain (Table 1).
The Tyr+Phe plasma availability ratio was significantly reduced after 2, 4, and 8 h by all treatments (see Supplementary Table 4). The Trp plasma availability ratio was significantly reduced by CGMP (1500 mg/kg)+Leu (300 mg/kg) and the BCAA mixture after 2 and 4 h, but not after 8 h, and was significantly increased by CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg) after 2 h, but not after 4 or 8 h. The DA and 5-HT tissue levels appeared to correlate with the plasma availability ratios of Tyr+Phe and Trp respectively, but a reduced Tyr+Phe availability ratio outlasted lowered DA brain levels, which were not significantly affected after 8 h.
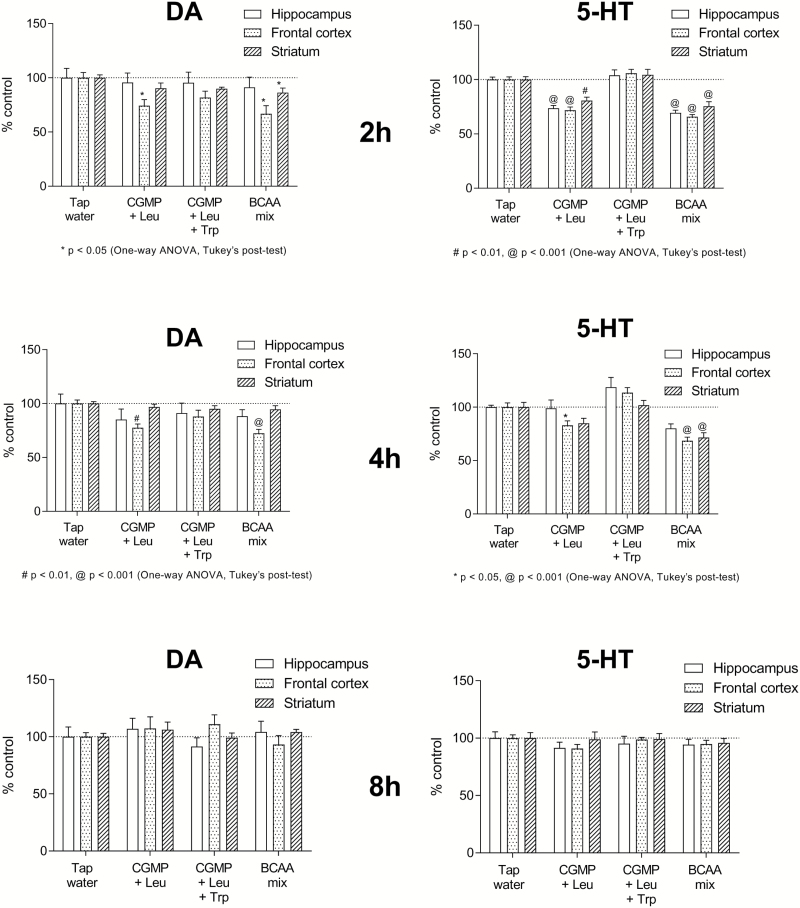
In agreement with the effects observed in Experiment 1, CGMP+Leu and the BCAA mixture induced a modest but significant decrease in DA levels in the frontal cortex after 2 h (respectively 74.4% and 66.9% of control, P<.05) and remained significant at 4 h after treatment (respectively 77.5% and 72.4% of control, P<.01) (Figure 2). 5-HT levels were depleted by CGMP+Leu and the BCAA mixture in the hippocampus, frontal cortex, and striatum after 2 h (P<.01), started to return to baseline at 4 h, and normalized after 8 h (Figure 2). NE remained relatively unaffected by the treatments; however, there was a tendency for the treatments to increase NE levels in the striatum, which was significant after 2 h (P<.05) (Supplementary Figure 1).
Figure 2.
The time-dependent effects on DA and 5-HT levels in the hippocampus, frontal cortex, and striatum by tap water, CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg), or BCAA mixture (Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg) at 2, 4 or 8 h after the oral gavage treatments. Values are expressed as percent of tap water control (mean±SEM). 1-way ANOVA followed by Tukey’s posthoc; *P<.05, #P<.01, @P<.001.
The levels of several other AAs were altered by the treatments used in the study (Table 1): Leu, Ile, and Val levels were elevated by the BCAA mixture in plasma and brain, whereas CGMP+Leu increased the levels of Thr (Table 1), which is abundant in CGMP as can be seen in Table 1. In addition, His, which is lacking in CGMP, was reduced in plasma and brain (Table 1).
The ANOVA results for Experiment 2 are provided in Supplementary Table 2.
Experiment 3: Behavioral Effects of CGMP
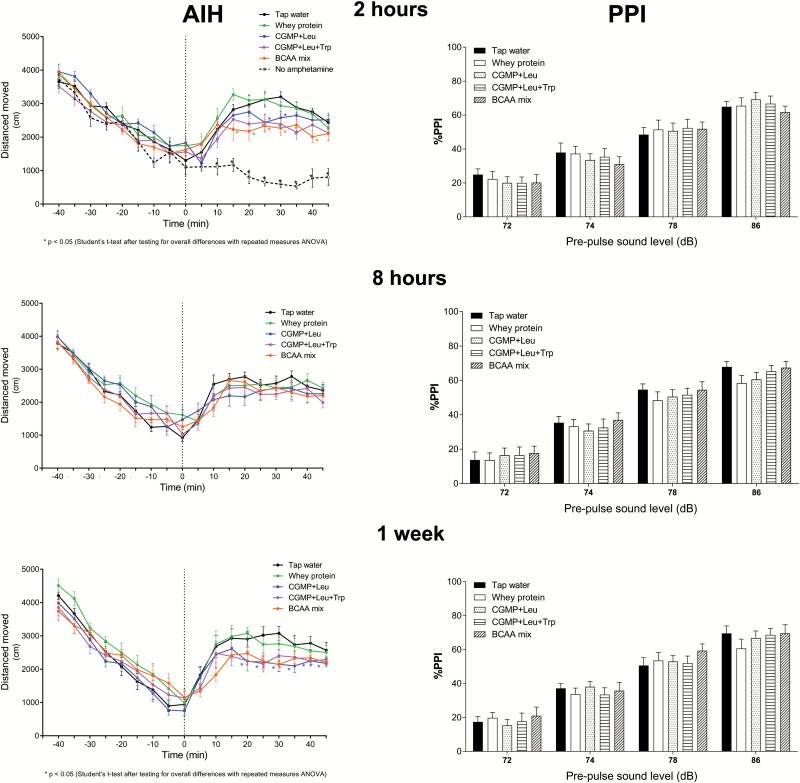
AIH
Between-subjects repeated ANOVAs revealed significant overall treatment effects on distance moved at 2 h after acute treatment (F(4, 45)=2.925, P=.031) and after 1 week of chronic treatment (F(4, 45)=3.016, P=.028) following the amphetamine injection (Figure 3). The overall treatment effect after 8 h was not significant (F(4, 45)=0.576, P=.682). The 2-h treatment group injected with saline was excluded from the ANOVA. Before the amphetamine injection, there were no significant overall treatment effects at 2 h (F(4, 45)=0.849, P=.502) or 8 h (F(4, 45)=1.527, P=.211) after acute treatment. However, there was a significant overall treatment effect before amphetamine administration after 1 week of chronic treatment (F(4, 45)=2.895, P=.032).
Figure 3.
The time-dependent effects on the behavioral responses of rats exposed to the AIH or PPI tests by tap water, CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg), BCAA mixture (Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg), or whey protein (1500 mg/kg). Independent groups of rats were exposed to the AIH or PPI after 2 h, 8 h, or 1 week (daily) of oral gavage treatment. Values are expressed as the mean±SEM. 1-way ANOVA followed by Tukey’s posthoc, *P<.05.
PPI
There were no significant overall treatment effects on %PPI at 2 h (F(4, 54)=0.183, P=.946) or 8 h (F(4, 52)=0.602, P=.663) after acute treatment, or after 1 week of chronic treatment (F(4, 53)=0.332, P=.855). A 1-way ANOVA comparing the mean %PPI of all treatments at different dB levels revealed significant differences at 2 h (F(3, 232)=101.59, P<.001) and 8 h after acute treatment (F(3, 224)=134.55, P<.001) and after 1 week of chronic treatment (F(3, 236)=118.31, P<.001).
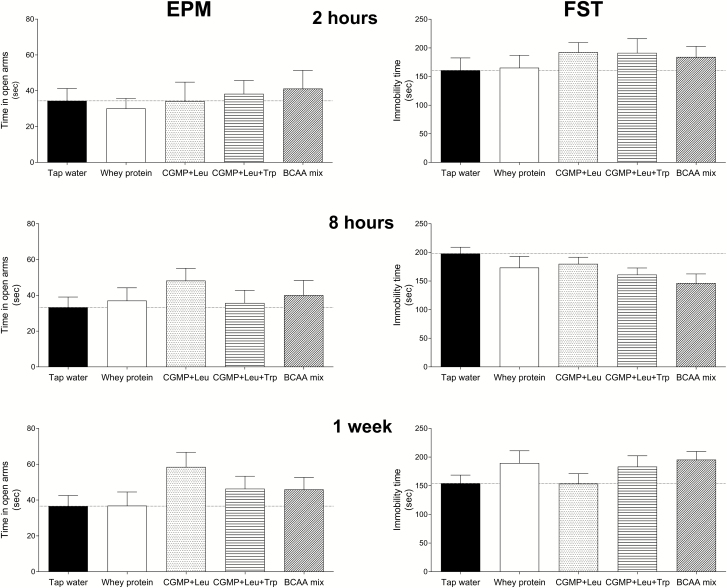
EPM
There were no significant overall treatment effects on the time spent in the open arms at 2 h (F(4, 55)=0.257, P=.904) or 8 h (F(4, 54)=0.668, P=.617) after acute treatment, or after 1 week of chronic treatment (F(4, 55)=1.580, P=.193). Although not significant, CGMP+Leu increased the time spent in the open arms by 45% at 8 h following an acute treatment (Figure 4).
Figure 4.
The time-dependent effects on the behavioral responses of rats exposed to the EPM or FST tests by tap water, CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg), BCAA mixture (i.e. Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg), or whey protein (1500 mg/kg). Independent groups of rats were exposed to the EPM or FST after 2 h, 8 h, or 1 week (daily) of oral gavage treatment. Values are expressed as the mean±SEM. 1-way ANOVA followed by Tukey’s posthoc, P>0.05.
FST
There were no overall treatment effects on immobility at 2 h (F(4, 54)=0.509, P=.730) or 8 h (F(4, 52)=1.994, P=.109) after acute treatment, or after 1 week of chronic treatment (F(4, 53)=1.269, P=.294). Although not significant, CGMP+Leu+Trp (P=.032) and the BCAA mixture (P=.014) reduced immobility time respectively by 18% and 26% at 8 h after acute treatment.
OFT
There were no significant overall treatment effects on the distance moved at 2 h (4, 55)=1.804, P=.141) or 8 h (F(4, 52)=1.830, P=.137) after acute treatment or after 1 week of chronic treatment (F(4, 54)=1.836, P=.135).
Discussion
The main finding in the present work is that CGMP induced a time-dependent reduction in Tyr, Trp, and Phe in brain and plasma, which was augmented by supplementing with Leu. CGMP+Leu reduced DA in the frontal cortex and 5-HT in the hippocampus, frontal cortex, and striatum after 2 and 4 h. CGMP+Leu also had antimanic activity in the amphetamine-induced hyperlocomotion test at 2 h after a single acute treatment and after 1 week of chronic treatment.
Theoretically, there are at least 2 possible mechanisms through which CGMP, Phe/Tyr/Trp-free AA mixtures, and BCAA mixtures may induce the depletion of central Phe, Tyr, and Trp. Firstly, the ingested AAs (especially Leu) would stimulate peripheral protein synthesis leading to the residual AAs that are absent in the mixture (i.e., Phe, Tyr, and/or Trp) to become depleted in plasma. Secondly, Phe, Tyr, and Trp use the same transporter and therefore compete for entry into the brain with other LNAAs (His, Ile, Leu, Met, Thr, and Val), and their relatively lower plasma levels causes them to be outcompeted and further reduced in the brain. The rate-limiting enzymes that synthesize DA and 5-HT, tyrosine hydroxylase and tryptophan hydroxylase respectively, are unsaturated under normal physiological conditions, and the rate of monoamine synthesis is therefore vulnerable to changes in Tyr and Phe availability.
In this study, we found that the CGMP+Leu- and BCAA mixture-induced depletion of Phe, Tyr, and Trp induced an antimanic-like effect in the AIH test at the same time-point at which Tyr, Phe, Trp, and DA levels in the brain are reduced, namely at 2 h, but not at 8 h after a single oral dose, although the Tyr+Phe plasma availability ratio was still significantly reduced 8 h after treatment. After 2 h, DA was reduced in frontal cortex by CGMP+Leu, whereas DA was reduced in the frontal cortex and striatum by the BCAA mixture. One other study has also observed an antimanic-like effect in the AIH test after acute i.p. administration of a BCAA mixture (Le Masurier et al., 2006). That the antimanic effect was still present when CGMP+Leu was supplemented with Trp and thereby prevented 5-HT depletion, suggests that it may have been mediated by a decrease in DA rather than 5-HT. This is despite the observation that the depletion of 5-HT was more pronounced than that of DA. NE levels remained relatively unaffected by the treatments in frontal cortex and hippocampus, with a trend for increased NE observed in striatum for the depleting treatments (see Supplementary Figure 1). These results suggest that NE levels in the brain regions investigated here may be less sensitive to Tyr and Phe depletion than DA.
Daily treatment with CGMP+Leu (±Trp) for 1 week had a cumulative antimanic-like effect, since the effect in the AIH test was present at 8 h after the final dose, a time-point that was devoid of any effect after a single treatment. A 1-week chronic treatment group was not included for the AA and monoamine analysis, and therefore it is not possible to correlate the antimanic-like activity after chronic treatment to AA and monoamine levels. However, the activity at 8 h post-gavage may be related to sustained altered amino acid availability after chronic treatment, or to secondary effects induced by sustained fluctuations in amino acid availability over a 1-week period. It therefore appears that CGMP may have potential for chronic antimanic use, although supplementing with natural protein at some meals would be needed to ensure that their nutritional requirements of all essential amino acids are met over longer periods of treatment. It is important to note that while only the BCAA mixture significantly lowered striatal DA, AIH was also significantly reduced by CGMP (1500 mg/kg)+Leu (300 mg/kg) acutely at 2 h and 8 h after chronic treatment. Therefore, this study does not provide a clear correlation between tissue DA levels and AIH.
The results from the EPM produced some interesting results. At 8 h after a single dose, there was a trend for CGMP+Leu to induce an anxiolytic-like effect, but not at 2 h (Figure 4). However, after 1 week of daily treatment (8 h after the final dose), a Student’s t test revealed a significant increase in the time spent in the open arms, suggesting that CGMP+Leu may exert an anxiolytic-like effect. Nonetheless, unlike its antimanic-like activity, it appeared that its anxiolytic-like action may have been mediated to a greater degree by 5-HT depletion, since Trp supplementation appeared to suppress the effect. This is in contrast to clinical studies that found that depletion of Trp by consuming Trp-free AA mixtures increased anxiety scores (Klaassen et al., 1998; Monteiro-dos-Santos et al., 2000; Argyropoulos et al., 2004). This said, the anxiolytic potential of CGMP warrants further investigation.
We did not observe any significant behavioral effects of any of the treatments on the FST after depletion of Phe+Tyr and/or 5-HT. This was not unexpected, since previous studies investigating Trp depletion did not report changes in the FST (van Donkelaar et al., 2010), although one study did find that chronic Trp depletion by a low-Trp diet increased immobility in the rat FST after 14 d but not after 7 d (Franklin et al., 2012). As mentioned in the Introduction, Trp depletion also does not appear to affect mood in healthy subjects (Benkelfat et al., 1994). As far as we are aware, the effect of Phe and Tyr depletion has not been investigated in the rodent FST as yet, whereas Phe and Tyr depletion studies in humans did not find notable changes in mood, although reward and cognition was reported to be negatively affected (McLean et al., 2004; McTavish et al., 2005; Roiser et al., 2005).
We did not observe notable effects in the PPI test, commonly used to identify schizophrenia-related behavior (Figure 3). The effects of dietary depletion of Phe+Tyr and/or 5-HT on PPI in rodents have not been investigated to our knowledge. However, a clinical study has shown that selective Phe+Tyr depletion had no effect, whereas selective Trp depletion and combined Phe+Tyr+Trp depletion led to a reduction in PPI in healthy humans (Mann et al., 2008). Our results did not confirm these findings, but given the known role of DA and 5-HT in the pathophysiology of schizophrenia (Geyer et al., 2001; Dean, 2003; Laruelle et al., 2003), alterations in monoamine function through dietary depletion of the precursor AAs cannot be ruled out.
In addition to the depletion of Phe, Tyr, and Trp, CGMP could theoretically also deplete other AAs it lacks by activating protein synthesis (see Table 1). As can be seen in Table 2, His was depleted by CGMP+Leu±Trp at 2 and 4 h after a single dose. This AA is the precursor for His, which is known to play a critical role in wakefulness and has also been associated with a number of clinical disorders in which cognitive performance is impaired (reviewed in Onodera et al., 1994). A study that investigated the effects of dietary His depletion in humans found that although its depletion impaired psychomotor performance, wakefulness was not affected (van Ruitenbeek et al., 2009).
In conclusion, this study shows that CGMP (supplemented with Leu) administered orally to rats induces a robust but transient depletion of Phe, Tyr, and Trp in plasma and brain tissue, lowers the levels of DA and 5-HT in the brain, and induces an antimanic-like effect. However, the treatments did not lower brain tissue DA without also lowering tissue 5-HT, and therefore the study does not distinguish between DA- and 5-HT-induced effects. It should be noted that the results presented here are circumstantial evidence for a mechanistic link between DA and 5-HT levels in the brain and an antimanic effect, and caution should therefore be exercised when making conclusions based solely on these results. The reductions observed in total tissue DA levels were marginal, and experiments measuring extracellular as opposed to total tissue neurotransmitter levels may give a more detailed picture of the mechanisms involved. Nevertheless, preclinical and clinical studies to investigate the potential of CGMP as an adjunctive treatment for mania and possibly also other psychiatric conditions are warranted.
Supplementary Items
Supplementary Table 1. The ANOVA results for treatment effects on plasma and brain levels of dopamine, serotonin, tyrosine, and tryptophan in Experiment 1.
Supplementary Table 2. The ANOVA results for treatment effects on plasma and brain levels of neurotransmitters, tryptophan, tyrosine, other large neutral amino acids, as well as Tyr+Phe and Trp plasma availability ratios in Experiment 2.
Supplementary Table 3. Tap water control mean values for neurotransmitters, tryptophan, tyrosine, and other large neutral amino acids in Experiment 2, expressed as mean±SEM µg/g tissue (brain tissue) or as mean±SEM µM (plasma).
Supplementary Table 4. The effects of treatment on the plasma availability ratios of tyrosine+phenylalanine (Tyr+Phe) and tryptophan (Trp) expressed as the availability ratio and as the availability ratio as percent of tap water control. The mean plasma concentrations (µM) of amino acids for the different treatment group were used to calculate the availability ratios according to using the following equations: Tyr+Phe availability=([Tyr] + [Phe]) / ([Trp] + [LNAAs]), and Trp availability=[Trp] / ([Tyr] + [Phe] + [LNAAs]), where [LNAAs] is the sum of the concentrations of the other large neutral amino acids histidine (His), isoleucine (Iso), leucine (Leu), methionine (Met), threonine (Thr), and valine (Val). Red and green values indicate a significant decrease or increase in the ratio, respectively, as calculated by 1-way ANOVAs followed by Tukey’s posthoc, P<.05.
Supplementary Figure 1. The time-dependent effects on NE levels in the hippocampus, frontal cortex, and striatum by tap water, CGMP (1500 mg/kg)+Leu (300 mg/kg), CGMP (1500 mg/kg)+Leu (300 mg/kg)+Trp (45 mg/kg) or BCAA mixture (i.e. Leu 600 mg/kg+Ile 450 mg/kg+Val 450 mg/kg). Samples were collected at 2, 4, or 8 h after the oral gavage treatments. Values are expressed as % of tap water control (mean±SEM).
Statement of Interest
Gregers Wegener declares having received lecture/consultancy fees from H. Lundbeck A/S, Servier SA, Astra Zeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd, and Pfizer Inc., Shire A/S, HB Pharma A/S, Arla Foods A.m.b.A., Alkermes Inc, and Mundipharma International Ltd.; and research funding from the Danish Medical Research Council, Aarhus University Research Foundation (AU-IDEAS initiative (eMOOD)), the Novo Nordisk Foundation, the Lundbeck Foundation, and EU Horizon 2020 (ExEDE). Erik Jensen is a full-time employee of Arla Foods Ingredients P/S. All other authors declare that they have no conflict of interest.
Acknowledgments
The authors thank Per Mikkelsen for his invaluable practical help in this study. This work was funded through an unrestricted grant from Arla Foods Ingredients P/S, and through Aarhus University Research Foundation (eMOOD initiative).
References
- Applebaum J, Bersudsky Y, Klein E(2007)Rapid tryptophan depletion as a treatment for acute mania: a double-blind, pilot-controlled study. Bipolar Disord 9:884–887. [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Hood SD, Adrover M, Bell CJ, Rich AS, Nash JR, Rich NC, Witchel HJ, Nutt DJ(2004)Tryptophan depletion reverses the therapeutic effect of selective serotonin reuptake inhibitors in social anxiety disorder. Biol Psychiatry 56:503–509. [DOI] [PubMed] [Google Scholar]
- Badawy A.(2013)Novel nutritional treatment for manic and psychotic disorders: a review of tryptophan and tyrosine depletion studies and the potential of protein-based formulations using glycomacropeptide. Psychopharmacology (Berl) 228:347–358. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A(2005)Epidemiology of bipolar disorders. Epilepsia 46:8–13. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN(1994)Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry 51:687–697. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP(2002)Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behav Brain Res 132:135–143. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Newbould E, Jaskiw GE(2008)Tyrosine depletion lowers dopamine synthesis and desipramine-induced prefrontal cortex catecholamine levels. Brain Res 1190:39–48. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Kyser AN, Jaskiw GE(2012)Tyrosine depletion lowers in vivo DOPA synthesis in ventral hippocampus. Eur J Pharmacol 696:70–76. [DOI] [PubMed] [Google Scholar]
- Brodnik Z, Double M, Jaskiw GE(2013)Presynaptic regulation of extracellular dopamine levels in the medial prefrontal cortex and striatum during tyrosine depletion. Psychopharmacology (Berl) 227:363–371. [DOI] [PubMed] [Google Scholar]
- Dean B.(2003)The cortical serotonin2a receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem 85:1–13. [DOI] [PubMed] [Google Scholar]
- Dionex Corporation (2015)Automated in-needle derivatization applying a user-defined program for the thermo scientific Dionex WPS-3000 split-loop autosampler. Technical Note 107, LPN 1197, 2001, Sunnyvale, CA. [Google Scholar]
- Fischer CW, Liebenberg N, Elfving B, Lund S, Wegener G(2012)Isolation-induced behavioural changes in a genetic animal model of depression. Behav Brain Res 230:85–91. [DOI] [PubMed] [Google Scholar]
- Franklin M, Bermudez I, Murck H, Singewald N, Gaburro S(2012)Sub-chronic dietary tryptophan depletion–an animal model of depression with improved face and good construct validity. J Psychiatr Res 46:239–247. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR(2001)Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156:117–154. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK(2007)Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology 32:1321–1333. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Mandrup KR, Kjaer SL, Bøgh IB, Rosenberg R, Wegener G(2011)Gestational chronic mild stress: effects on acoustic startle in male offspring of rats. Int J Dev Neurosci 29:495–500. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Kirkbride B, Bongiovanni R(2006)In rats chronically treated with clozapine, tyrosine depletion attenuates the clozapine-induced in vivo increase in prefrontal cortex dopamine and norepinephrine levels. Psychopharmacology (Berl) 185:416–422. [DOI] [PubMed] [Google Scholar]
- Jauhar S, Nour MM, Veronese M, Rogdaki M, Bonoldi I, Azis M, Turkheimer F, McGuire P, Young AH, Howes OD(2017)A test of the transdiagnostic dopamine hypothesis of psychosis using positron emission tomographic imaging in bipolar affective disorder and schizophrenia. JAMA Psychiatry 74:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, King SM(2001)Influence of circadian phase and test illumination on pre-clinical models of anxiety. Physiol Behav 72:99–106. [DOI] [PubMed] [Google Scholar]
- Kirkedal C, Wegener G, Moreira F, Joca SRL, Liebenberg N(2017)A dual inhibitor of FAAH and TRPV1 channels shows dose-dependent effect on depression-like behaviour in rats. Acta Neuropsychiatr 29:324–329. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Klumperbeek J, Deutz NE, van Praag HM, Griez E(1998)Effects of tryptophan depletion on anxiety and on panic provoked by carbon dioxide challenge. Psychiatry Res 77:167–174. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, van Someren A, Deutz NE, Honig A, van Praag HM(1999)Mood effects of 24-hour tryptophan depletion in healthy first-degree relatives of patients with affective disorders. Biol Psychiatry 46:489–497. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A(2003)Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158. [DOI] [PubMed] [Google Scholar]
- Le Masurier M, Houston G, Cowen P, Grasby P, Sharp T, Hume S(2004)Tyrosine-free amino acid mixture attenuates amphetamine-induced displacement of [11C]raclopride in striatum in vivo: a rat PET study. Synapse 51:151–157. [DOI] [PubMed] [Google Scholar]
- Le Masurier M, Oldenzeil W, Lehman C, Cowen P, Sharp T(2006)Effect of acute tyrosine depletion in using a branched chain amino-acid mixture on dopamine neurotransmission in the rat brain. Neuropsychopharmacology 31:310–317. [DOI] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, Gunn R, Young SN, Benkelfat C(2004)Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: A PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 29:427–432. [DOI] [PubMed] [Google Scholar]
- Liebenberg N, Harvey BH, Brand L, Brink CB(2010)Antidepressant-like properties of phosphodiesterase type 5 inhibitors and cholinergic dependency in a genetic rat model of depression. Behav Pharmacol 21:540–547. [DOI] [PubMed] [Google Scholar]
- Liebenberg N, Joca S, Wegener G(2015)Nitric oxide involvement in the antidepressant-like effect of ketamine in the flinders sensitive line rat model of depression. Acta Neuropsychiatr 27:90–96. [DOI] [PubMed] [Google Scholar]
- Mann C, Croft RJ, Scholes KE, Dunne A, O’Neill BV, Leung S, Copolov D, Phan KL, Nathan PJ(2008)Differential effects of acute serotonin and dopamine depletion on prepulse inhibition and p50 suppression measures of sensorimotor and sensory gating in humans. Neuropsychopharmacology 33:1653–1666. [DOI] [PubMed] [Google Scholar]
- McLean A, Rubinsztein JS, Robbins TW, Sahakian BJ(2004)The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 171:286–297. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Cowen PJ, Sharp T (1999a) Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology (Berl) 141:182–188. [DOI] [PubMed] [Google Scholar]
- McTavish SF, McPherson MH, Sharp T, Cowen PJ (1999b) Attenuation of some subjective effects of amphetamine following tyrosine depletion. J Psychopharmacol 13:144–147. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Raumann B, Cowen PJ, Sharp T (2001a) Tyrosine depletion attenuates the behavioural stimulant effects of amphetamine and cocaine in rats. Eur J Pharmacol 424:115–119. [DOI] [PubMed] [Google Scholar]
- McTavish SF, McPherson MH, Harmer CJ, Clark L, Sharp T, Goodwin GM, Cowen PJ (2001b) Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. Br J Psychiatry 179:356–360. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Mannie ZN, Harmer CJ, Cowen PJ(2005)Lack of effect of tyrosine depletion on mood in recovered depressed women. Neuropsychopharmacology 30:786–791. [DOI] [PubMed] [Google Scholar]
- Monteiro-dos-Santos PC, Graeff FG, dos-Santos JE, Ribeiro RP, Guimarães FS, Zuardi AW(2000)Effects of tryptophan depletion on anxiety induced by simulated public speaking. Braz J Med Biol Res 33:581–587. [DOI] [PubMed] [Google Scholar]
- Onodera K, Yamatodani A, Watanabe T, Wada H(1994)Neuropharmacology of the histaminergic neuron system in the brain and its relationship with behavioral disorders. Prog Neurobiol 42:685–702. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C(2014)Paxino’s and Watson’s The rat brain in stereotaxic coordinates, 7th ed Amsterdam; Boston: Elsevier/Academic Press. [Google Scholar]
- Pereira M, Andreatini R, Schwarting RK, Brenes JC(2014)Amphetamine-induced appetitive 50-khz calls in rats: a marker of affect in mania?Psychopharmacology (Berl) 231: 2567–2577. [DOI] [PubMed] [Google Scholar]
- Roiser JP, McLean A, Ogilvie AD, Blackwell AD, Bamber DJ, Goodyer I, Jones PB, Sahakian BJ(2005)The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology 30:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarna A, Gijsman HJ, McTavish SF, Harmer CJ, Cowen PJ, Goodwin GM(2003)Effects of a branched-chain amino acid drink in mania. Br J Psychiatry 182:210–213. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF(2012)Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014. [DOI] [PubMed] [Google Scholar]
- Tohen M, Jacobs TG, Feldman PD(2000)Onset of action of antipsychotics in the treatment of mania. Bipolar Disord 2:261–268. [DOI] [PubMed] [Google Scholar]
- Van der Does AJ.(2001)The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord 64:107–119. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, Blokland A, Lieben CK, Kenis G, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J(2010)Acute tryptophan depletion in C57BL/6 mice does not induce central serotonin reduction or affective behavioural changes. Neurochem Int 56:21–34. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Sambeth A, Vermeeren A, Young SN, Riedel WJ(2009)Effects of L-histidine depletion and L-tyrosine/L-phenylalanine depletion on sensory and motor processes in healthy volunteers. Br J Pharmacol 157:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson MC, Bell EC, Dave S, Asghar SJ, McGrath BM, Silverstone PH(2005)Valproate attenuates dextroamphetamine-induced subjective changes more than lithium. Eur Neuropsychopharmacol 15:633–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.