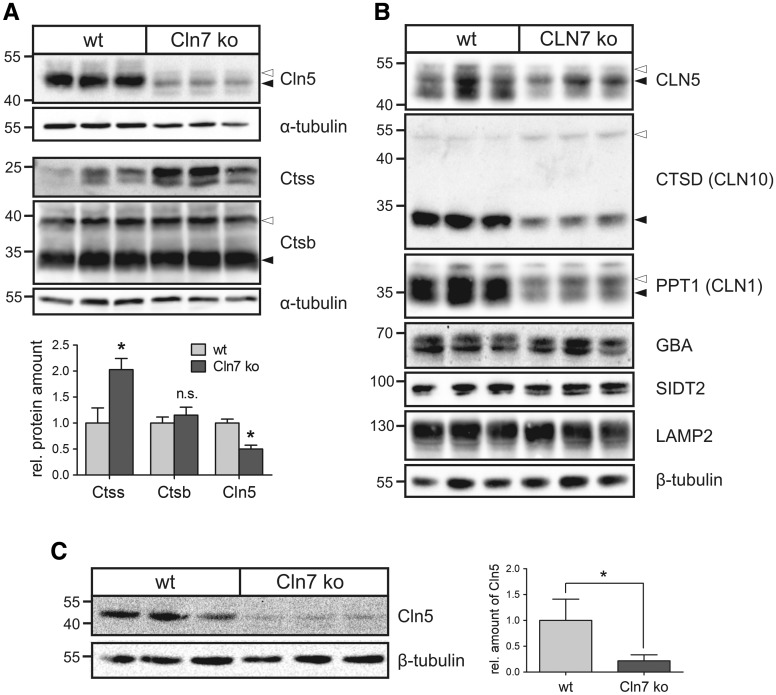
Figure 3.
Decreased Cln5 protein amounts in Cln7 ko cells and brain tissue. (A) Primary bone macrophages isolated from 5-month-old Cln7 ko and age-matched wild-type mice (N = 3) were cultivated for 14 days. Whole cell lysates were analysed by immunoblotting using antibodies against Cln5, cathepsins S (Ctss) and B (Ctsb). Equal loading was verified by α-tubulin western blotting. The positions of the molecular mass markers and the precursor (open arrowhead) and mature (filled arrowhead) Cln5 and Ctsb proteins, respectively, are indicated. Densitometric quantification of the immunoreactive band intensities has been performed and the relative protein amounts are shown in a bar diagram (mean ± SD, n = 3–5). n.s.: not significant, *P < 0.05 (two-tailed Student’s t-test). (B) Lysosome-enriched fractions of CLN7 ko and wild-type HAP1 cells were analysed by western blotting using antibodies against CLN5, cathepsin D (CTSD), PPT1, glucocerebrosidase (GBA), SIDT2 and LAMP2. Equal loading was confirmed by α-tubulin western blotting. The positions of the molecular mass markers and the precursor (open arrowhead) and mature (filled arrowhead) forms of CLN5, CTSD and PPT1, respectively, are indicated. (C) Whole brain lysates from three 10-month-old Cln7 ko and age-matched wild-type mice were analysed by Cln5 immunoblotting. β-Tubulin western blotting was used as loading control. The positions of the molecular mass markers are indicated. Bar diagram represents densitometric analysis of Cln5 protein levels normalized to the loading control (mean ± SD, n = 5). *P < 0.05 (two-tailed Student’s t-test).