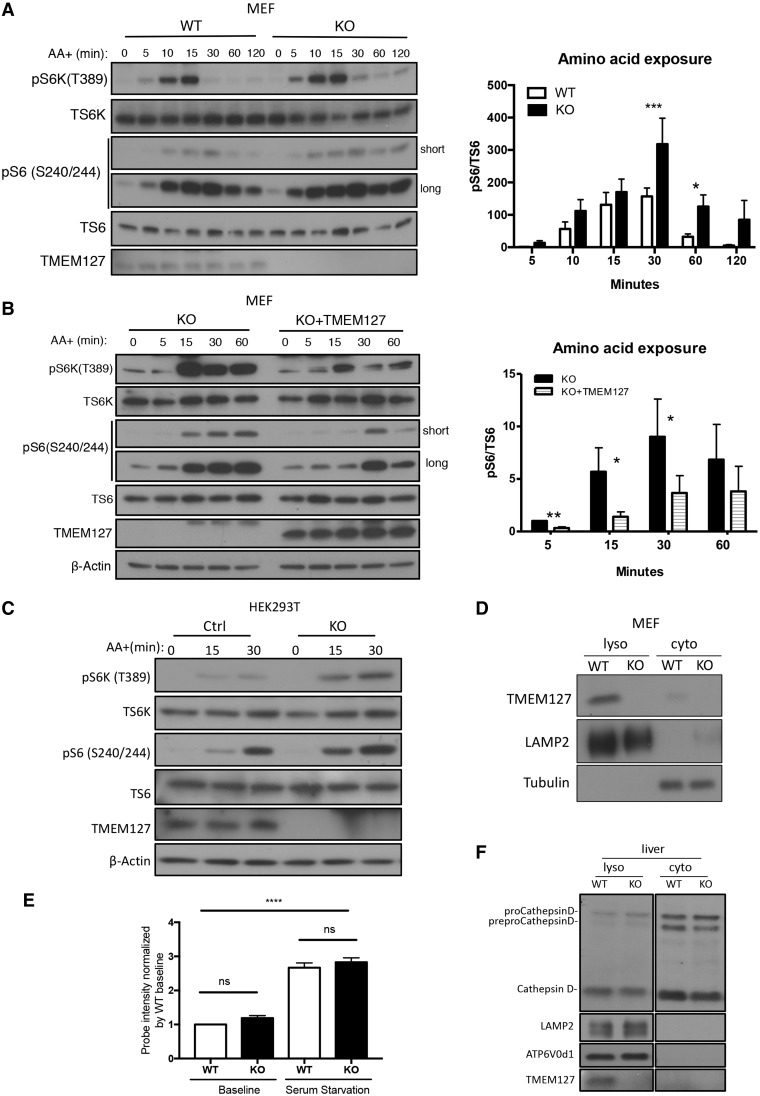
Figure 1.
Endogenous TMEM127 resides in lysosomal-enriched fractions and affects mTORC1 activation in response to amino acids. (A) Western blot of WT or Tmem127 KO MEFs cultured under amino acid deprivation for 2 h and re‐exposed to amino acids (AA+) in identical concentration as RPMI media for the designated times; blots were probed with antibodies detecting phosphorylated S6 kinase (p‐S6K T389) and S6 (p‐S6 S240/244), their corresponding total protein (T‐S6K and T‐S6, respectively) and TMEM127. Long and short exp, long and short exposure of blot. Quantification was performed using Image J; bar graphs display mean ± SEM; statistical significance was calculated from five biological replicates using two-way ANOVA with Bonferroni’s post-test, P-values are as follows: *P <0.05; ***P < 0.005 (results from an independent experiment shown in Supplementary Material, Fig. S1A). (B) Western blot of immortal Tmem127 KO MEF stably expressing an empty MSCV retrovirus (KO) or a TMEM127‐expressing MSCV (KO+ TMEM127), treated as in (A) and probed with phosphorylated S6 kinase (p‐S6K T389) and S6 (p‐S6 S240/244), their corresponding total protein (T‐S6K and T‐S6, respectively), TMEM127 and β‐actin antibodies. Quantification was performed using Image J; bar graphs display mean ± SEM; statistical significance was determined from three biological replicates using two-way ANOVA with Bonferroni’s post-test, P-value is as follows: *P <0.05 (results from an independent experiment shown in Supplementary Material, Fig. S1B). (C) Western blot of Control (Ctrl) and TMEM127 KO HEK293FT cells generated by CRISPR-Cas9 editing, starved of amino acid for 2 h and re-exposed to amino acids in identical concentration to those of RPMI media for the designated times. Blots were probed with antibodies detecting phosphorylated S6 kinase (p‐S6K T389) and S6 (p‐S6 S240/244), their corresponding total protein (T‐S6K and T‐S6, respectively), TMEM127 and β‐actin antibodies. Three biological replicates were performed. (D) Western blot of fractionated Tmem127 WT and KO MEF lysates showing detection of endogenous TMEM127 in membrane fraction containing lysosomes (lyso) cell fractions but not in cytosolic (cyto) fractions, LAMP2 and β‐tubulin are lysosomal and cytosolic markers, respectively. More than five biological replicates were performed. (E) Fluorescence levels of the Lysosensor‐DND‐189TM probe in four independent WT and Tmem127 KO MEF pairs, measured by flow cytometry at baseline culture conditions or after overnight serum starvation; each MEF pair was normalized to its respective baseline WT fluorescence, bar graphs display mean ± SEM. Statistical significance was determined from four biological replicates using one-way ANOVA with Tukey’s post-test, P-value is as follows: ****P < 0.001, n.s., nonsignificant. (F) Western blot of lysosomal and cytosolic fractions from WT and KO mouse liver probed with a CathepsinD antibody that detects precursor (preproCathepsinD), intermediate (proCathepsinD) and mature forms of CathepsinD; TMEM127 indicates genotype, LAMP2 and α-tubulin serve as lysosomal and cytosol markers, respectively. Data were obtained from three independent sets of mice.