Abstract
We asked, is uric acid as effective as proteinuria at identifying perinatal risk in high-risk women with gestational hypertension? Uric acid was measured in samples obtained ≈4.6 weeks predelivery in 259 women with prior preeclampsia from the National Institute of Child Health and Human Development network study of low-dose aspirin to prevent preeclampsia. Participants were grouped according to the presence/absence of gestational hypertension (H), proteinuria (P), and hyperuricemia (U). Adverse perinatal outcomes were not different between H or U and women with normal values (normal blood pressure, urinary protein, and uric acid [NNN]). Preterm birth was greater in hypertension and proteinuria (HP) and hypertension and hyperuricemia (HU) compared to NNN (relative risk [RR] = 2.4, P = .03 and 3.8, P < .01), respectively. In addition, in HU women, delivery was earlier (36.6 ± 3.4 vs 38.4 ± 2.3 weeks, P < .001) and small for gestational age infants <fifth centile more frequent (RR = 8.2, P = .01) compared to NNN women. This study sought to determine if uric acid is as effective as proteinuria at identifying perinatal risk in high-risk women with gestational hypertension. Our results suggest that hyperuricemia is at least as accurate as proteinuria at identifying perinatal risk in high-risk women with gestational hypertension.
Keywords: hyperuricemia, proteinuria, gestational hypertension, preeclampsia, risk
Introduction
The risk that new-onset hypertension in pregnancy presents to a mother and her baby has been conventionally differentiated by the presence or absence of proteinuria.1 The presence of proteinuria in women with new-onset gestational hypertension is clinically diagnosed as preeclampsia1 and is associated with increased maternal/neonatal morbidity and mortality compared to isolated gestational hypertension.2 However, the exclusive use of proteinuria to define the risk that is associated with new-onset hypertension in pregnancy has recently been questioned. Several studies have shown that proteinuria is not the sole predictor of adverse maternal and neonatal outcomes.3–5 In light of these findings, The American College of Obstetrics and Gynecology Task Force on Hypertension in Pregnancy has recommended that other systemic abnormalities (eg, thrombocytopenia and impaired liver function) are sufficient to diagnose preeclampsia in the absence of proteinuria.1 Because evidence has also demonstrated that the quantity of proteinuria predicts neither fetal nor maternal risk,5 the task force has also removed heavy proteinuria from the list of features that are used to classify/diagnose severe preeclampsia.1
Uric acid is commonly elevated in the blood of women with preeclampsia and may represent a marker that is equally as effective as proteinuria at identifying perinatal risk in women with gestational hypertension.3,4 Uric acid, a product of purine degradation, is mainly produced in the liver and primarily excreted by the kidneys.6,7 In women with preeclampsia, it has been demonstrated that uric acid levels become elevated prior to the presence of proteinuria and elevated blood pressure (elevated as early as 10 weeks’ gestation).8 Several mechanisms, including decreased renal clearance, increased tissue breakdown, and increased xanthine oxidase/dehydrogenase activity, are suggested as potential mechanisms responsible for increased circulating uric acid.6 An in-depth review of uric acid in the setting of preeclampsia is provided by Bainbridge and Roberts.6
Moreover, in 2 separate studies of low-risk women, we found that the presence of hyperuricemia in women with gestational hypertension was associated with increased risk of preterm birth and delivery of a small for gestational age (SGA) infant compared to women with isolated gestational hypertension.3,4 Furthermore, the perinatal risk profile associated with hyperuricemia and gestational hypertension was found to be as great as the perinatal risk profile associated with proteinuria and gestational hypertension in the study by Roberts et al.3 Both studies also found that the presence of hyperuricemia in women with preeclampsia (gestational hypertension and proteinuria [HP]) was associated with increased risk of adverse perinatal outcomes compared to women with preeclampsia and normal uric acid values.3,4
In the present study, we asked whether uric acid was as effective as proteinuria at identifying perinatal risk in women with gestational hypertension that were part of a high-risk cohort. To answer this question, we examined a data set comprised of a high-risk group of women with prior preeclampsia that were enrolled in the National Institute of Child Health and Human Development (NICHD) study of low-dose aspirin to prevent preeclampsia.9 We also asked whether the measurement of uric acid might be a reasonable substitute for proteinuria in identifying women with new-onset pregnancy hypertension who are at increased risk of adverse perinatal outcome (preterm birth, SGA less than the 10th centile, and SGA less than the 5th centile).
Materials and Methods
Study Population
This study was a secondary analysis of data from the NICHD network study, “Low-dose aspirin to prevent preeclampsia in women at high risk.”9 Using a retrospective cohort study design, uric acid was measured in blood samples that were available for 259 women enrolled in the high-risk subgroup of women with prior preeclampsia (n = 130 prescribed aspirin; n = 129 prescribed placebo). Of the 259 subjects with prior preeclampsia, 13.1% developed preeclampsia (HP) in their subsequent pregnancy. The predelivery blood samples used in the current study were the final samples drawn and were obtained an average of 4.6 weeks before delivery. The original study was approved by institutional review boards (IRBs) at all centers, and the use of samples for the current study was approved by the University of Pittsburgh IRB.
Pregnancy Outcome Classifications
In the NICHD study,9 preeclampsia was defined as a blood pressure of ≥140 mm Hg systolic or ≥ 90 mm Hg diastolic on at least 2 occasions at least 4 hours apart after 20 weeks of gestation in association with proteinuria of ≥2+ (urine dipstick) on 2 occasions at least 4 hours apart or a 24-hour urine collection of >300 mg of protein in the absence of urinary tract infection. Women were excluded from the NICHD study if they had hypertension prior to pregnancy or before 20 weeks of gestation. For the current study, hyperuricemia was defined as a serum uric acid concentration of ≥1 standard deviation of the mean for the gestational age at which the samples were obtained (eg, 38-week gestation: uric acid value ≥5.46 mg/dL would indicate U).10 Uric acid was measured at Magee-Womens Research Institute (Pittsubrgh, Pennsylvania) with the Sigma Diagnostics assay (Procedure No. 685). The interassay variability was 9.2%. The outcome of the pregnancy was unknown to the individual performing the assay.
For the current study, subjects were grouped into 1 of the 8 groups according to the presence or absence of hypertension (H), proteinuria (P), and hyperuricemia (U). These groups was classified as follows: (1) H (hypertension), (2) HP (hypertension and proteinuria), (3) HPU (hypertension, proteinuria, and hyperuricemia), (4) HU (hypertension and hyperuricemia), (5) P (proteinuria), (6) PU (proteinuria and hyperuricemia), (7) U (hyperuricemia), and (8) NNN (women with normal blood pressure, urinary protein, and uric acid).
The adverse perinatal outcomes of interest included preterm birth and SGA. Preterm birth was defined as delivery prior to 37 weeks and 0 days of gestation. Small for gestational age was defined as less than the 10th centile or the 5th centile for gestational age (SGA 10th or SGA 5th).11
Statistical Analysis
Demographic variables were compared using the chi-square test or Fisher exact test. Gestational age across diagnostic groups was assessed using the Kruskal-Wallis test. Log-binomial regression was used to calculate the relative risks associated with the diagnostic groups on the risk of preterm birth and SGA infants. Linear regression was used to assess the association of the diagnostic groups with gestational age at delivery and birth weight centile. A nominal P value <.05 (2 sided) was considered to indicate statistical significance. No adjustments were made for multiple comparisons. Analyses were performed using SAS (SAS Institute, Cary, North Carolina) and StatXact (Cytel Software, Cambridge, Massachusetts) software.
Results
No Association Between Aspirin Treatment and Uric Acid Concentration
As previously reported, aspirin was ineffective to prevent preeclampsia in the NICHD study.9 We further determined that treatment with aspirin was not significantly associated with differences in uric acid in this current study (P = .84). Therefore, the treatment and placebo-treated groups were combined for this analysis. Isolated proteinuria (P) occurred twice, and proteinuria with hyperuricemia (PU) occurred once. These 2 groups were not included in the analysis.
Demographic Characteristics
In the 6 groups we compared (NNN, H, U, HP, HU, and HPU), we found no differences in maternal age, marital status, education, BMI, or maternal age distributions (Table 1). The proportion of smokers did vary across the 6 groups (P = .02), with the greatest proportion of smokers in the HPU group (44%).
Table 1.
Demographic Characteristics.a
| NNN (n = 131) | H (n = 25) | U (n = 34) | HP (n = 34) | HU (n = 16) | HPU (n = 16) | P | |
|---|---|---|---|---|---|---|---|
| Race | .47 | ||||||
| White, non-Hispanic | 24 (18) | 9 (36) | 6 (18) | 8 (24) | 3 (19) | 5 (31) | |
| White, Hispanic | 7 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | |
| Black, non-Hispanic | 100 (76) | 16 (64) | 28 (82) | 26 (76) | 12 (75) | 11 (69) | |
| BMI | .49 | ||||||
| <18.5 | 6 (5) | 0 (0) | 2 (6) | 1 (3) | 0 (0) | 0 (0) | |
| 18.5-<25 | 60 (46) | 6 (24) | 9 (26) | 10 (29) | 4 (25) | 6 (38) | |
| 25-<30 | 28 (22) | 8 (32) | 10 (29) | 9 (26) | 4 (25) | 4 (25) | |
| ≥30 | 36 (28) | 11 (44) | 13 (38) | 14 (41) | 8 (50) | 6 (38) | |
| Smoking | 24 (18) | 2 (8) | 5 (15) | 2 (6) | 1 (6) | 7 (44) | .024 |
| Education, years | .97 | ||||||
| <12 | 35 (27) | 6 (24) | 8 (24) | 8 (24) | 4 (25) | 6 (38) | |
| 12 | 69 (53) | 13 (52) | 16 (47) | 19 (56) | 10 (63) | 7 (44) | |
| >12 | 27 (21) | 6 (24) | 10 (29) | 7 (21) | 2 (13) | 3 (19) | |
| Maternal age, years | .69 | ||||||
| <18 | 5 (4) | 1 (4) | 1 (3) | 2 (6) | 1 (6) | 1 (6) | |
| 18-21 | 45 (34) | 9 (36) | 8 (24) | 8 (24) | 4 (25) | 4 (25) | |
| 22-24 | 30 (23) | 3 (12) | 10 (29) | 7 (21) | 2 (13) | 3 (19) | |
| 25-29 | 30 (23) | 3 (12) | 9 (26) | 12 (35) | 5 (31) | 3 (19) | |
| 30-34 | 15 (11) | 7 (28) | 5 (15) | 3 (9) | 4 (25) | 3 (19) | |
| ≥35 | 6 (5) | 2 (8) | 1 (3) | 2 (6) | 0 (0) | 2 (13) | |
| Married | 36 (27) | 11 (44) | 9 (26) | 11 (32) | 4 (25) | 9 (56) | .15 |
Abbreviations: NNN, normal blood pressure, urinary protein, and uric acid; H, hypertension; U, hyperuricemia; HP, hypertension and proteinuria; HU, hypertension and hyperuricemia; HPU, hypertension, proteinuria, and hyperuricemia; BMI, body mass index.
aData are presented as n (%).
Uric Acid Distributions by Diagnostic Group
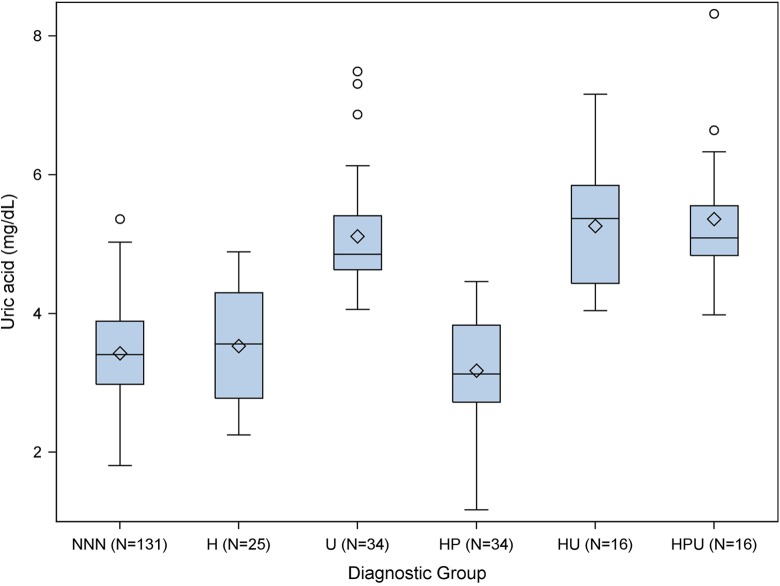
We measured serum uric acid concentrations in samples obtained, on average, 4.6 weeks prior to delivery. The distribution of uric acid concentrations by diagnostic group (NNN, H, U, HP, HU, and HPU) is presented in Figure 1. The gestational age at sampling was similar across the different diagnostic groups (P = .18).
Figure 1.
Uric acid distributions by diagnostic group. Diamond = mean, line within box = median, bottom of box = 25th centile, top of box = 75th centile, bottom whisker = minimum or 1.5 × IQR (interquartile range) if outliers, top whisker = maximum or 1.5 × IQR if outliers, and outliers = circles (values >1.5 × IQR).
H and U Versus NNN
In this group of high-risk women, the presence of isolated gestational hypertension or hyperuricemia was not associated with adverse birth outcomes. Average gestational age at delivery, average birth weight centile, and frequency of SGA (<5th centile and <10th centile) was similar in women with isolated gestational hypertension (H) or hyperuricemia (U) compared to women with normal values (NNN; Tables 2 and 3). Although the frequency of preterm birth was slightly higher among H (20.0%) and U (17.6%) women compared to NNN women (9.9%), these differences were not statistically significant (H: P = .14; U: P = .21; Table 2).
Table 2.
Associations Between Diagnostic Groups and Gestational Age at Delivery and Risk of Preterm Birth (<37 Weeks).a
| Diagnostic Group | Gestational Age at Delivery, weeks | Birth < 37 Weeks | |||
|---|---|---|---|---|---|
| Mean ± Std | P | N (%) | RR (95% CI) | P | |
| NNN | 38.4 ± 2.3 | N/A | 13 (9.9) | 1.0 (referent) | N/A |
| H | 38.1 ± 2.0 | .50 | 5 (20.0) | 2.0 (0.8-5.2) | .14 |
| U | 38.6 ± 1.7 | .75 | 6 (17.6) | 1.8 (0.7-4.3) | .21 |
| HP | 37.8 ± 2.0 | .18 | 8 (23.5) | 2.4 (1.1-5.3) | .034 |
| HU | 36.3 ± 3.4 | <.001 | 6 (37.5) | 3.8 (1.7-8.5) | .001 |
| HPU | 36.6 ± 3.3 | .003 | 8 (50.0) | 5.0 (2.5-10.3) | <.001 |
Abbreviations: NNN, normal blood pressure, urinary protein, and uric acid; H, hypertension; U, hyperuricemia; HP, hypertension and proteinuria; HU, hypertension and hyperuricemia; HPU, hypertension, proteinuria, and hyperuricemia; Std, standard deviation; N/A, not applicable; RR, relative risk; CI, confidence interval.
aOther contrasts of interest for birth <37 weeks are as follows (RR [(95% CI], P value): HPU versus HP (2.1 [1.0-4.6], P = .06), HU versus HP (1.6 [0.7-3.8], P = .30), and HU versus H (1.9 [0.7-5.1], 0.22).
Table 3.
Associations Between Diagnostic Groups and Birth Weight Centile and Risk of SGA.a
| Birth Weight Centile | SGA < 5th Centile | SGA < 10th Centile | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± Std | P | N (%) | RR (95% CI) | P | N (%) | RR (95% CI) | P | |
| NNN | 50.5 ± 27.9 | N/A | 3 (2.3) | 1.0 (referent) | N/A | 9 (6.9) | 1.0 (referent) | N/A |
| H | 54.9 ± 26.1 | .47 | 1 (4.0) | 1.7 (0.2-16.1) | .62 | 1 (4.0) | 0.6 (0.1-4.4) | .60 |
| U | 50.3 ± 27.6 | .96 | 1 (3.0) | 1.3 (0.1-12.3) | .81 | 1 (3.0) | 0.4 (0.1-3.4) | .43 |
| HP | 60.4 ± 28.2 | .07 | 1 (2.9) | 1.3 (0.1-12.0) | .83 | 2 (5.9) | 0.9 (0.2-3.8) | .84 |
| HU | 46.1 ± 32.9 | .54 | 3 (18.8) | 8.2 (1.8-37.2) | .007 | 3 (18.8) | 2.7 (0.8-9.1) | .10 |
| HPU | 33.6 ± 20.2 | .022 | 1 (6.3) | 2.7 (0.3-24.7) | .37 | 2 (12.5) | 1.8 (0.4-7.7) | .42 |
Abbreviations: NNN, normal blood pressure, urinary protein, and uric acid; H, hypertension; U, hyperuricemia; HP, hypertension and proteinuria; HU, hypertension and hyperuricemia; HPU, hypertension, proteinuria, and hyperuricemia; Std, standard deviation; SGA, small for gestational age; RR, relative risk; CI, confidence interval.
aOther contrasts of interest for birth weight centile are as follows: HU versus H (P = .32), HU versus HP (P = .09), and HPU versus HP (P < .01). Other contrasts of interest for SGA < 5th centile are as follows: RR (95% CI), P value]: HPU versus HP [2.1 (0.1-31.9), P = .59), HU vs HP [6.4 (0.7-56.6), P = .10], HU vs H [4.7 (0.5-41.2), 0.16]. Other contrasts of interest for SGA < 10th centile are as follows (RR [95% CI], P value): HPU versus HP (2.1 [0.3-13.8], P = .43), HU versus HP (3.2 [0.6-17.2], P = .18), and HU versus H (4.7 [0.5-41.2], P = .16).
HP Versus NNN
The diagnosis of preeclampsia has been traditionally based on the presence of both gestational hypertension and proteinuria (without consideration of hyperuricemia). When comparing the high-risk group of women with hypertension and proteinuria but normal uric acid concentration (HP) to women with all normal values (NNN), we found that the average gestational age at delivery, average birthweight centile, and frequency of SGA (<5th centile and <10th centile) were similar between HP and NNN women (Tables 2 and 3). Preterm birth was significantly more common in HP women than NNN women (P = .03, Table 2).
HU Versus NNN
Although isolated gestational hypertension or isolated hyperuricemia was not associated with increased frequency of adverse fetal outcomes in high-risk women, this was not the case for women with both gestational hypertension and hyperuricemia (HU). The combined presence of gestational hypertension and hyperuricemia was associated with poorer perinatal outcomes compared to women with normal values (NNN). On average, HU women delivered 2 weeks earlier (36.3 ± 3.4 vs 38.4 ± 2.3 weeks, P < .001), had 3.8 times greater risk of preterm birth (95% confidence interval, CI [1.7-8.5], P < .01), and had 8.2 times greater risk of delivering a SGA infant less than the fifth centile (95% CI [1.8-37.2], P = .01; Tables 2 and 3). Although average birth weight centile was slightly lower among HU women (46.1 ± 32.9) compared to NNN women (50.5 ± 27.9), these differences were not statistically significant (P = .54, Table 3).
HU Versus H
We also investigated whether the presence of hyperuricemia influenced fetal outcomes in women with gestational hypertension by comparing women with gestational hypertension and hyperuricemia (HU) to women with isolated gestational hypertension (H). On average, HU women delivered 1.8 weeks earlier than H women (36.3 ± 3.4 vs 38.1 ± 2.0, P = .02). In HU women, the frequencies of preterm birth and SGA (both <5th and <10th centiles) were higher, and the average birth weight centile was lower, compared to H women, but these differences were not statistically significant (P values ≥.16; Tables 2 and 3).
HU Versus HP
To examine whether hyperuricemia was as effective as proteinuria at identifying gestational hypertensive pregnancies that are associated with increased fetal morbidity, we next compared HU to HP women. As presented in Table 2, HU women delivered more than a week earlier (36.3 vs 37.8, P = .03). They also had increased frequencies of preterm birth and SGA, along with lower average birth weight centile, compared to HP women (Table 3); however, the differences did not reach statistical significance (P values ≥.09).
HPU Versus HP
We further sought to explore the potential influence that the presence of hyperuricemia in women with preeclampsia (HPU) had on fetal outcome. Compared to HP women (gestational hypertension and proteinuria only), HPU women delivered, on average, 1 week earlier (36.6 vs 37.8) and had increased frequencies of both preterm birth (50.0% vs 23.5%) and SGA (<10th centile: 12.5% vs 5.9%; <5th centile: 6.3% vs 2.9%); however, these differences were not statistically significant (P values ≥.06; Tables 2 and 3). Average birth weight centile was significantly lower in HPU compared to HP women (P < .01).
Does Uric Acid Identify at Risk Pregnancies as Well as Proteinuria?
To begin to investigate whether the measurement of uric acid by itself (protein not determined) would identify at risk pregnancies with gestational hypertension as effectively as determining urinary protein without knowledge of uric acid, we combined HP and HPU groups of women (HP-all) and HU and HPU groups of women (HU-all) and then compared each with NNN group of women (Table 4). Because the HP-all and HU-all groups were not mutually exclusive, we could not statistically compare these groups; however, compared with NNN pregnancies, both the HU-all and HP-all groups displayed similarities in perinatal risk profiles. Compared with NNN pregnancies, both HU-all and HP-all women delivered earlier and had increased frequency of preterm birth. Comparison of the HU-all group to the NNN group also revealed that the frequency of SGA <5th centile was significantly higher in the HU-all group. This statistical difference was not observed between the HP-all and NNN groups.
Table 4.
Comparison of HP-all and HU-all to NNN.a
| NNN (n = 131) | HU-All (n = 32) | HP-All (n = 50) | |||
|---|---|---|---|---|---|
| P | P | ||||
| GA at delivery, weeks | 38.4 ± 2.3 | 36.5 ± 3.3 | <.001 | 37.4 ± 2.5 | .011 |
| Birth <37 weeks | 13 (9.9) | 14 (43.8) | <.001 | 16 (32.0) | <.001 |
| Birth weight centile | 50.5 ± 27.9 | 39.8 ± 27.6 | .051 | 51.8 ± 28.7 | .79 |
| SGA <5th centile | 3 (2.3) | 4 (12.5) | .028 | 2 (4.0) | .62 |
| SGA <10th centile | 9 (6.9) | 5 (15.6) | 0.15 | 4 (8.0) | 0.76 |
Abbreviations: HU-all, combined HU and HPU groups of women; HP-all, combined HP and HPU groups of women; GA, gestational age; SGA, small for gestational age; HU, hypertension and hyperuricemia; HPU, hypertension, proteinuria, and hyperuricemia; HP, hypertension and proteinuria; NNN, normal blood pressure, urinary protein, and uric acid.
aData are presented as mean ± standard deviation or N (%).
Discussion
The presence of hyperuricemia is common in women with preeclampsia and its presence has been associated with increased risk of preterm birth3,4 and the delivery of infants that are SGA.4 Evidence further suggests that the presence of hyperuricemia in women with gestational hypertension generates a perinatal risk profile that is at least equal to that associated with gestational hypertension and proteinuria.3 Despite these findings, the utility of uric acid measurement, along with the influence that hyperuricemia has on risk stratification and clinical management decisions in women with gestational hypertension and preeclampsia, remains controversial.12
In this study of high-risk women with prior preeclampsia, we asked whether uric acid was as useful as proteinuria to identify perinatal risk in women with gestational hypertension (Does the presence of hyperuricemia, like proteinuria, identify women with gestational hypertension as being at increased risk for adverse perinatal outcomes?). Overall, we found that hyperuricemia was at least as accurate as proteinuria in the identification of gestational hypertensive pregnancies at risk of increased fetal morbidity. Our comparison of HU-all and HP-all women to NNN women further suggests that the measurement of uric acid may represent a reasonable substitute for proteinuria in the identification of those gestational hypertensive pregnancies that are at increased risk of developing adverse outcomes. Moreover, our results in a high-risk sample heavily weighted toward black non-Hispanic women are comparable to low-risk studies heavily weighted toward white women. This consistency ultimately speaks to the generalizability of these findings and the potential utility of uric acid measurement across different races and varying degrees of prenatal risk.
Gestational Hypertension: Is Concomitant Hyperuricemia Associated With Fetal Outcomes?
The new onset of blood pressure elevations at or beyond 20 weeks’ gestation, in the absence of proteinuria, in most studies, is associated with maternal and fetal risk intermediate to the risks presented by normal pregnancy and preeclampsia.13 Studies from our group have been the exception, perhaps because our diagnosis of gestational hypertension requires the absence of hyperuricemia. As such, we have found that the fetal outcomes associated with isolated gestational hypertension (without hyperuricemia) are no different than with uncomplicated normotensive pregnancies.3 This is confirmed in the current study of high-risk women in whom gestational age at delivery, birth weight centile, and risk of preterm birth and SGA were similar among women with and without isolated gestational hypertension. Important to note is that women with isolated hyperuricemia (n = 34), like women with isolated gestational hypertension, also had similar fetal outcomes compared to women with normotensive pregnancies.
However, gestational hypertension coupled with hyperuricemia appears to identify a group of women with increased risk of adverse fetal outcomes.3,4 We found that women with gestational hypertension and hyperuricemia (HU) had poorer perinatal outcomes, including increased risk of preterm birth and SGA, compared to normotensive women (NNN). These results are consistent with those reported in our study of low-risk women.3 This study supports the syndromic nature of preeclampsia where adverse outcomes are more common in women with multisystemic findings (hypertension and proteinuria or hyperuricemia) but not with isolated manifestions (eg, hypertension or hyperuricemia alone).
In a direct comparison of HU to H women, our previous studies found that the risk of preterm birth and SGA was also significantly increased in HU women compared to women with isolated gestational hypertension (H).3,4 We found a similar trend in this study (preterm birth 17.5% higher and SGA 14.8% higher in HU compared to H women; however, these differences in risk were not statistically significant likely due to the small sample size).
Overall, our results coupled with previous reports3,4 suggest that isolated gestational hypertension and gestational hypertension with hyperuricemia may represent different conditions that may warrant different management approaches. As such, the inclusion of uric acid measurement in the diagnostic workup of all women with gestational hypertension might help clinicians to identify which women with gestational hypertension are more likely to develop adverse outcomes and therefore require a higher level of surveillance.
Moreover, the presence or absence of hyperuricemia could impact the clinician’s decision concerning the timing of delivery. The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy currently recommends that women with mild gestational hypertension reaching thirty-seven 0/7ths weeks of gestation should be delivered.1 Our data would suggest that this recommendation is appropriate for those women with gestational hypertension and hyperuricemia given their increased risk of adverse perinatal outcomes, but perhaps not appropriate for those women with isolated gestational hypertension in whom risk was not increased. This should be tested in prospective assessments with sample sizes sufficient to determine positive and negative predictive values for the presence of elevated uric acid.
Is Hyperuricemia as Good a Delineator of Risk as Proteinuria?
The presence or absence of proteinuria has traditionally been used to delineate the risk that new-onset hypertension in pregnancy presents to a mother and her baby.1 But even in the absence of proteinuria, we have shown that women with gestational hypertension and hyperuricemia are at increased risk of adverse perinatal outcomes. Given this finding, the singular use of proteinuria as a marker of risk will likely not identify all women with gestational hypertension that are at increased risk of adverse perinatal outcomes.
We asked whether hyperuricemia, without knowledge of proteinuria, would be as good a delineator of risk as proteinuria, without knowledge of hyperuricemia, in a group of women with gestational hypertension. The comparison of HP-all (HP and HPU women) and HU-all (HU and HPU women) women to normotensive women (NNN) found that the association with increased risk of preterm birth and SGA was at least as strong, if not stronger, in women with elevated uric acid compared to women with proteinuria. This particular finding begs the following question: Could uric acid determination represent an alternative to the determination of urinary protein excretion? Again this merits investigation in a prospective study.
Problems With Urinary Protein Measurement
Despite the consistent use of urinary protein excretion as a marker of risk in women with gestational hypertension, the measurement of quantitative urinary protein excretion is problematic. Urinary protein excretion is not constant and can be affected by a myriad of factors, including posture, activity level, and blood pressure elevations.14 Moreover, the physiologic dilatation of the ureters during pregnancy can lead to urine retention, thereby introducing the potential for collection errors and inaccurate measurement of urinary protein excretion.14
There are also issues with the methods that are utilized to measure urinary protein excretion. The urine dipstick has been found to be associated with a high number of false-positive and false-negative results and may not correlate well with the 24-hour urine collection.1,14 The “gold standard” 24-hour urine collection is often inconvenient and has been found to be an inaccurate measure of urinary protein excretion in pregnancy.15 Although the spot urine protein/creatinine ratio may avoid the timing issues surrounding the 24-hour urine collection, the cutoff value used to indicate an abnormal protein/creatinine ratio has varied from study to study.14
In addition to the problems associated with the measurement of quantitative urinary protein excretion, mounting evidence across multiple studies demonstrates that the level of proteinuria is a poor predictor of maternal and fetal risk.5 Given these findings, American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy has also removed heavy proteinuria from the list of features that are used to classify/diagnose severe preeclampsia.1 As such, if determination of quantitative urinary protein excretion is problematic, and the level of proteinuria is not predictive of adverse outcomes, perhaps we should consider using alternative markers.
Advantages of Uric Acid Measurement
It appears that uric acid elevation is at least as accurate as urinary protein excretion in identifying risk in pregnant women with new-onset hypertension. Furthermore, the use of uric acid measurement as a delineator of risk in women with gestational hypertension has several advantages over the determination of quantitative urinary protein excretion. Unlike the 24-hour urine collection, uric acid concentration can be measured by a simple, inexpensive blood test that does not require a timed collection. Moreover, research suggests that, unlike quantity of urinary protein excretion, increasing uric acid concentrations are predictive of increasing adverse perinatal outcomes.3 In a study of low-risk women, the odds of preterm birth increased as uric acid concentrations increased in women with gestational hypertension ± proteinuria. In women with gestational hypertension, the odds of SGA also increased as the concentration of uric acid increased.3 Taken together, the advantages of uric acid measurement and the documented disadvantages of quantitative urinary protein excretion suggest additional studies to determine whether routine uric acid determination might reasonably replace routine determination of urinary protein excretion.
Limitations
Although this was the first study, to our knowledge, to ask whether uric acid was as good as proteinuria at delineating risk in a group of high-risk women, the small sample size of several comparison groups represents a significant limitation. Isolated proteinuria (P) occurred twice, and proteinuria with hyperuricemia (PU) occurred once. As a result, these 2 groups were not included in the analysis, which prevented us from fully assessing the risk that is associated with hyperuricemia. For the remaining 6 comparison groups, small sample numbers may have influenced our ability to detect statistically significant differences when they truly existed. We did not adjust our P values for multiple comparisons due to controversy over how to choose the appropriate adjustment; several prominent statisticians16 argue against any adjustment for multiple comparisons because of the spurious nature of such decisions, but it is worth noting that we had 6 comparison groups and 5 different outcomes analyzed in this article, so P values should be interpreted with that understanding. Moreover, we acknowledge that the study may have been underpowered, resulting in the possibility of type II error in which we failed to reject a false null hypothesis. Ideally, follow-up studies in high-risk women with much larger sample sizes are needed to validate our findings. Finally, uric acid was measured in samples that were obtained on average 4.6 weeks before delivery. Since uric acid levels increase with gestational age and worsening disease,3,7,8 the associations that we did find, including potentially borderline associations and trends may have been strengthened if we had used samples that were drawn closer to delivery. Furthermore, because of small numbers we only examined fetal outcomes. Although maternal adverse outcomes do not increase with increasing proteinuria, it is possible that threshold proteinuria indicates maternal risk (eg, eclampsia) better than hyperuricemia.
Conclusion
In summary, this study of high-risk women found that hyperuricemia was at least as accurate as proteinuria in the identification of gestational hypertensive pregnancies at risk of increased fetal morbidity, thereby confirming studies in low-risk women. Accumulating evidence across multiple studies suggests that the inclusion of uric acid measurement into clinical practice, perhaps instead of urinary protein, has the potential to improve the clinician’s ability to identify at risk women, which could ultimately impact perinatal outcomes. However, additional follow-up studies with larger sample sizes are first needed to validate these findings and to examine maternal risk to demonstrate the diagnostic capabilities of uric acid prior to the implementation of any practice changes.
Acknowledgments
The authors would like to thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network for providing the data for this secondary analysis.
Authors’ Note: The work was completed at Magee-Womens Research Institute (Pittsburgh, Pennsylvania). The contents of this report represent the views of the authors and do not represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported, in part, by National Institute of Health Grants F32NR014622 and P01HD030367.
References
- 1. American College of Obstetricians and Gynecologiests Task Force on Hypertension in Pregnancy. Hypertens Pregnancy. Washington, DC: The American College of Obstetricians and Gynecologists; 2013. [Google Scholar]
- 2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 3. Roberts JM, Bodnar LM, Lain KY, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46(6):1263–1269. [DOI] [PubMed] [Google Scholar]
- 4. Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484–492. [DOI] [PubMed] [Google Scholar]
- 5. Thangaratinam S, Coomarasamy A, O’Mahony F, et al. Estimation of proteinuria as a predictor of complications of pre-eclampsia: a systematic review. BMC Med. 2009;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(suppl A):S67–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conrad KP, Gaber LW, Lindheimer MD. The kidney in normal pregnancy and preeclamsia In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. San Diego, CA: Academic Press, Elsevier; 2009:297–334. [Google Scholar]
- 8. Powers RW, Bodnar LM, Ness RB, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194(1):160e.1–160.e8. [DOI] [PubMed] [Google Scholar]
- 9. Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705. [DOI] [PubMed] [Google Scholar]
- 10. Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91(2):128–132. [DOI] [PubMed] [Google Scholar]
- 11. Alexander GR, Kogan MD, Himes JH. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–231. [DOI] [PubMed] [Google Scholar]
- 12. Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS; Tests in Prediction of Pre-eclampsia Severity review group. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG. 2006;113(4):369–378. [DOI] [PubMed] [Google Scholar]
- 13. Myatt L, Redman CW, Staff AC, et al. Strategy for standardization of preeclampsia research study design. Hypertension. 2014;63(6):1293–1301. [DOI] [PubMed] [Google Scholar]
- 14. Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: the need for a more pathophysiological approach. Obstet Gynecol. 2010;115(2 pt 1):365–375. [DOI] [PubMed] [Google Scholar]
- 15. Cote AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199(6):625 e621–626. [DOI] [PubMed] [Google Scholar]
- 16. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]