Abstract
Objective:
Validate single versus sequential culture media for murine embryo development.
Design:
Prospective laboratory experiment.
Setting:
Assisted Reproduction Laboratory.
Animals:
Murine embryos.
Interventions:
Thawed murine zygotes cultured for 3 or 5 days (d3 or d5) in single or sequential embryo culture media developed for human in vitro fertilization.
Main Outcome Measures:
On d3, zygotes developing to the 8 cell (8C) stage or greater were quantified using 4’,6-diamidino-2-phenylindole (DAPI), and quality was assessed by morphological analysis. On d5, the number of embryos reaching the blastocyst stage was counted. DAPI was used to quantify total nuclei and inner cell mass nuclei. Localization of ubiquitin C-terminal hydrolase L1 (UCHL1) and ubiquitin C-terminal hydrolase L3 (UCHL3) was reference points for evaluating cell quality.
Results:
Comparing outcomes in single versus to sequential media, the odds of embryos developing to the 8C stage on d3 were 2.34 time greater (P = .06). On d5, more embryos reached the blastocyst stage (P = <.0001), hatched, and had significantly more trophoblast cells (P = .005) contributing to the increased total cell number. Also at d5, localization of distinct cytoplasmic UCHL1 and nuclear UCHL3 was found in high-quality hatching blastocysts. Localization of UCHL1 and UCHL3 was diffuse and inappropriately dispersed throughout the cytoplasm in low-quality nonhatching blastocysts.
Conclusions:
Single medium yields greater cell numbers, an increased growth rate, and more hatching of murine embryos. Cytoplasmic UCHL1 and nuclear UHCL3 localization patterns were indicative of embryo quality. Our conclusions are limited to murine embryos but one might speculate that single medium may also be more beneficial for human embryo culture. Human embryo studies are needed.
Keywords: embryo, culture, media, UCHL1, UCHL3
Introduction
A variety of different types of human embryo culture media for in vitro fertilization (IVF) have been developed over decades, often using animal embryos (rat, mouse, and pig) to optimize culture media for embryonic growth and development. Media may include proteins, amino acids, carbohydrates, salts, and other components playing roles in, for example, the regulation of embryo metabolism, maintenance of intracellular osmolality, and redox potential.1–14 In the 1950s and 1960s, pioneers of in vitro embryo culture developed media mimicking the in vivo oviduct environment.9–11 Whitten discovered that murine embryos require glucose to develop past the 8 cell (8C) stage in vitro10 and that 2C mouse embryos cleaved into blastocysts by including lactate in the medium but only after the late 2C period.11 Numerous media components interact causing elevated complexity in development of different types of culture media. For example, nonessential amino acids and glutamine in media diminish glucose toxicity on preimplantation mammalian embryos and lactate, while beneficial in combination with amino acids and pyruvate is toxic when present by itself at levels ranging from 10 to 20 mmol/L.6,12–14
Today there are numerous types of commercially available human embryo culture media. Single medium and sequential media are 2 approaches used by many human IVF clinics. Single medium, as the name implies, contains the same components for the entire culture period reducing embryo manipulation potentially caused by transfer to new medium outside of the incubator. Sequential media uses 2 media with different components aimed at mimicking the environment of the human reproductive tract to support the evolving needs of the developing embryo as it makes it way to the uterus via the oviduct. The quality of human embryos developing in single medium versus sequential culture media, however, remains controversial.15–19
Greatly needed clinically useful biomarkers for embryo quality and development are lacking. Ubiquitin C-terminal hydrolase L1 (UCHL1) and ubiquitin C-terminal hydrolase L3 (UCHL3) are 2 deubiquitinating enzymes (DUBs) regulating the oocyte/embryo ubiquitin–proteasome system by targeting and cleaving covalent ubiquitin–ubiquitin and ubiquitin–substrate protein bonds.20,21 In porcine embryos, UCHL1 regulates function and maturation of oocyte and embryo cortices whereas UCHL3 localizes and is involved in normal function of oocyte meiotic spindles. Morula compaction and blastocyst formation do not occur in Uchl1 gad−/− mutant females.21
The purpose of the present study was to determine the quality and developmental attributes of embryos grown in commercially available single medium versus sequential media over a period of 5 days using murine embryos. We hypothesized that single culture medium, by maintaining a constant environment, improves embryo development in vitro compared to sequential media. We used UCHL1/UCHL3 cytoplasmic/nuclear localization patterns to facilitate identification and quality assessment of blastocyst embryos.
Materials and Methods
Embryo Culture Media
Two types of culture media were compared. One was a single culture medium (Global, LifeGlobal, Guilford, Connecticut) with protein containing α-ß globulins (LifeGlobal Protein Supplement, LGPS 050, Soundview Road, Guilford, CT 06437, USA) designed to support human embryo growth and development from the zygote to the blastocyst stage. The other was a sequential media system (Irvine Scientific, Santa Ana, California) where preimplantation 1 medium (P-1) was designed to maintain growth and development of human zygotes to the 8C stage and Multiblast medium was designed for development from the 8C stage to the blastocyst stage. Both P-1 and Multiblast media included a protein substitute (Serum Substitute Supplement, Irvine Scientific). Prior to use, all media were equilibrated overnight in a CO2 incubator (HeraCell 150i, Thermo Fisher Scientific, Palm Beach, Florida) with 6% CO2, at 37°C and >98% humidity producing a pH of 7.3 and osmolality of 270 to 290 mOsm. Media components and osmolality are shown in Table 1.
Table 1.
Human Embryo Culture Media.a,b
| Components | P-1 (Step 1) | Multiblast (Step 2) | Global |
|---|---|---|---|
| Sodium bicarbonate | X | X | X |
| Phenol Red | X | X | X |
| Sodium pyruvate | X | X | X |
| Taurine | X | X | |
| Gentamycin | X | X | |
| Gentamicin sulfate | X | ||
| Sodium chloride | X | X | X |
| Calcium chloride | X | X | X |
| Potassium chloride | X | X | X |
| Sodium citrate | X | X | |
| Sodium lactate | X | X | X |
| Magnesium sulfate | X | X | X |
| Potassium phosphate | X | X | |
| Glucose | X | X | |
| EDTA | X | ||
| Alanyl glutamine | X | X | |
| Alanine | X | X | |
| Asparagine | X | X | |
| Aspartic acid | X | X | |
| Glutamic acid | X | X | |
| Glycine | X | X | |
| Proline | X | X | |
| Serine | X | X | |
| Arginine | X | X | |
| Cysteine | X | X | |
| Histidine | X | X | |
| Isoleucine | X | X | |
| Leucine | X | X | |
| Lysine | X | X | |
| Methionine | X | X | |
| Phenylalanine | X | X | |
| Threonine | X | X | |
| Tryptophan | X | X | |
| Tyrosine | X | X | |
| Valine | X | X | |
| Osmolality, mOsm | 290 | 290 | 270 |
aComponent concentrations of all media are proprietary.
bMedia are from Irvine Scientific (P-1 and Multiblast) and LifeGlobal (Global).
To equate the level of embryo manipulation for the 2 media types and help eliminate embryo manipulation as a factor affecting embryo development, the single medium was refreshed on day 3 (d3), which is divergent from the continuous 5 day culture per manufacturer’s protocol. This was done to parallel embryo manipulation with sequential media requiring transferring embryos from the first to second media on d3 to support outcomes most likely caused by the specific culture media rather than the embryo manipulation.
Embryo Culture
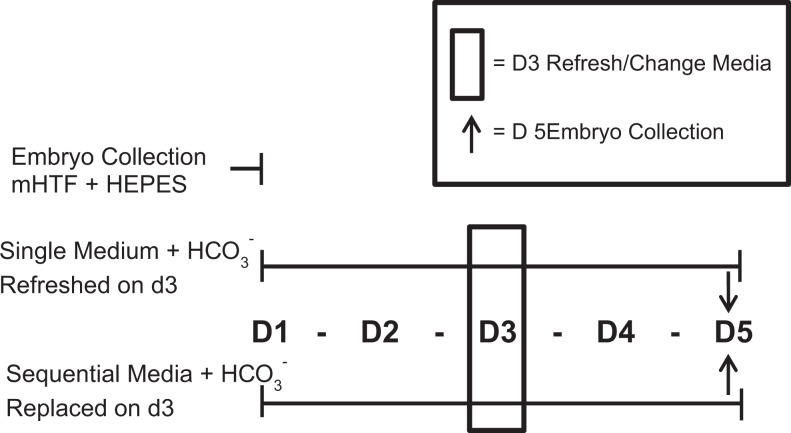
Murine zygotes (EmbryoTech; Haverhill, Massachusetts) collected from superovulated female B6C3F-1 and male B6D2F-1 mice were pooled and randomly allocated into ThawAlert straws (n = ∼20 to 22 per straws) for cryopreservation by the manufacturer. (No mice were used in this study, only the cryopreserved zygotes.) Two straws of zygotes (n = ∼20 per straw) were thawed in our laboratory in accordance with the EmbryoTech protocol, pooled, and randomly placed into 1 mL of equilibrated single medium or sequential media on day 1 (d1). Four total replicates were performed over a 6-month period for a totaling of ∼80 zygotes per media type. A replicate effect was incorporated into all statistical models. Figure 1 illustrates the experimental design of the murine embryo media experiments. Nonembryo toxic, light mineral oil (1 mL; Irvine Scientific) was used as a media overlay for both media.
Figure 1.
Experimental design. Thawed murine zygotes were pooled in collection media modified Human Tubal Fluid (mHTF) + 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The zygotes were randomly assigned to single (n = 60) or sequential (n = 60) media types. On day 3 (d3), single medium was refreshed, whereas sequential media were changed from P-1 to multiblast medium. Embryos were collected on d3 or day 5 (d5) and processed for analyses.
Murine embryos were cultured at the Missouri Center for Reproductive Medicine and Fertility, Department of Obstetrics, Gynecology and Women’s Health, University of Missouri. Both the single and sequential media embryo cultures were conducted using the same lot of double-well organ culture dishes (Falcon In Vitro Fertilization plastic ware; Corning Incorporated, Corning, New York), in the same incubators and in the same laboratory environment.
Embryo Assessment
Embryo development was evaluated on d3 at 1000 hours and on day 5 (d5) at 1330 hours. The numbers of embryos developing ≥ to the 8C stage on d3 or the blastocyst stage on d5 were quantified using an Olympus IX-71 inverted phase microscope with Hoffman contrast, equipped with an Olympus DP70 digital camera (Center Valley, Pennsylvania).
The total number of nuclei per blastocyst was determined by counting 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad, California; 12.5 μg/mL) stained nuclei (600× magnification). Nuclei were counted 3 times per blastocyst (600× magnification) by 2 different investigators blinded to the media type. The outcomes of counting nuclei were not different between the 2 investigators substantiating reliability in this method. The inner cell mass (ICM) was identified by general nuclear staining by DAPI and comparisons to differential contrast images and UCHL1 and UCHL3 localization patterns as reference points (400× and 600× magnification). The ICM nuclei were counted as described.
To determine the number of presumptive trophoblast (TE) cells per embryo, the mean number of ICM nuclei was subtracted from the mean total blastocyst nuclei count. Nuclear anomalies including fragmentation, pyknosis (shrinking/condensation), karyorrhexis (disintegration/granulation), and/or chromosomal misalignment were identified and quantified (200, 400, and 600× magnification). Multiple planes of focus enabled assessment of all nuclei. Abnormal nuclei were counted and compared as described for the total and ICM nuclear counts.
Hatching was determined by referencing fluorescent light and bright field micrographs. The number of hatching and completely hatched blastocysts was compared to the number of embryos without morphological signs of hatching between the 2 media groups.
UCHL1 and UCHL3 immunofluorescent localization
On d5, embryos were fixed in 2% formaldehyde in phosphate-buffered saline (PBS; pH 7.4) with 0.8% polyvinyl pyrrolidone to add viscosity (Irvine Scientific). After 40 minutes, the embryos were washed twice and stored at 4°C in PBS. Immunofluorescent localization of UCHL1 and UCHL3 was performed within 5 to 7 days after fixation.
Dual UCHL1 and UCHL3 immunofluorescent localization was performed in the Sutovsky laboratory as described previously and validated for oocytes, sperm, and preimplantation embryos.20–23 Briefly, the embryo zona pellucida was permeabilized using a 0.1% Triton X-100 PBS solution for 40 minutes, and nonspecific antibody binding was blocked with 0.1% Triton X-100 and 5% normal goat serum in PBS for 30 minutes. The embryos were then exposed to a mixture of primary antibodies including anti-UCHL1 mouse immunoglobulin (IgG; 2.5 μg/mL; Abcam, Cambridge, Massachusetts) and affinity purified rabbit anti-UCHL3 IgG (2.5 μg/mL; LifeSpan Biosciences/MBL, Woburn, Massachusetts) diluted in the same blocking buffer for 18 hours at 4°C. The following morning, embryos were washed in the same buffer and transferred to a secondary antibody solution.
Secondary antibody solutions included goat antimouse IgG conjugated to fluorescein isothiocyanate (GAR-FITC, Zymed, San Francisco, California; 2 μg/mL) and goat antirabbit IgG conjugated to tetramethyl rhodamine isothiocyanate (GAR-TRITC, UHCL3; Zymed; 2 μg/mL). Nuclear DNA was identified by DAPI staining as described. VectaShield mounting medium (Vector Labs, Burlingame, California) was used to affix the coverslips onto the glass slides.
The UHCL1 and UHCL3 staining was examined using a Nikon (Tallahassee, Florida) Eclipse 800 epifluorescence microscope with differential interface contrast and a Cool Snap CCD camera (Photometrics, Tucson, Arizona) with data analysis using MetaMorph Microscopy Automation & Image Analysis Software (Sunnyvale, California). The Cell Scoring Application Module was used to analyze all cells with the nuclear stain DAPI and targeted cells-of-interest stained for UCHL1 and UCHL3 using fluorophoreidentifies, GAR-FITC for UCHL1 and GAR-TRITC for UCHL3. We anticipated that in healthy embryos, UCHL1 would be associated with blastomere cortices and UCHL3 with the nuclear matrix of the blastomeres as described for other species.20,21 The combination of nuclear damage and diffuse UCHL1 and UCHL3 staining throughout the cytoplasm and the nuclei was deemed indicative of poor blastocyst developmental capacity.
Statistical Analysis
Estimates of total nuclei number, ICM nuclei number, and presumptive TE nuclei number between blastocysts were computed using a linear mixed model with least-squares means. Relevant comparisons were made using an approximate t test based on these estimates. Differences in nuclear counts between investigators were tested by one-way analysis of variance. Exact conditional logistic regression was used to model binary outcomes, such as development to the 8C stage, development to the blastocyst stage, hatching, abnormal nuclei, and TE nuclei. For such analyses the odds ratio (OR) is reported, along with the P value and the associated confidence interval (CI) for the OR. For all analyses, P < .05 was considered significant, and 95% confidence levels were used. Power analyses were performed for the sample sizes and all relevant tests were β ≥ 0.80. Finally, a replicate effect was incorporated into all of the models as a random effect to account for experiments analyzed in replicates. All models were fit using SAS 9.22 (SAS Institute Inc, Cary, North Carolina), specifically PROC MIXED or PROC LOGISTIC.
Results
Embryo development on d3 or d5
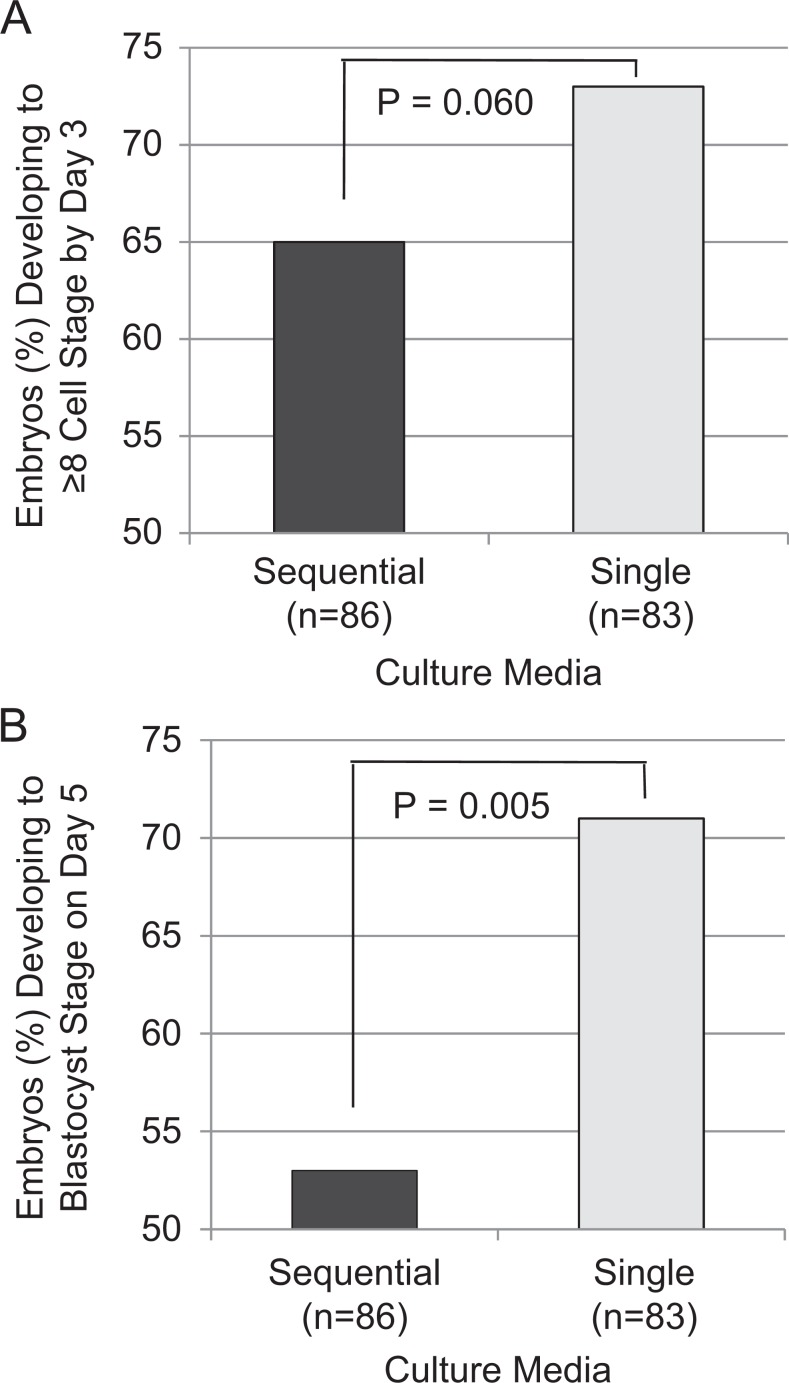
The odds of embryos developing to ≥ 8C stage on developmental d3 were more than 2 times greater in single medium than sequential media, approaching statistical significance (OR = 2.34, CI: 0.97-5.98, P = .060; Figure. 2A). The odds of observing a d5 blastocyst-stage embryo in single medium were more than 3 times greater than the odds of observing a d5 blastocyst-stage embryo in the sequential media (OR = 3.63, CI: 1.65-8.47, P = .005 and Figure. 2B).
Figure 2.
Embryo development on day 3 (d3) and day 5 (d5). A, On d3, the percentage of embryos ≥ 8 cell (8C) or < 8C developing in single culture media tended to be greater but did not reach statistical significance (P < .06). B, On d5, the percentage of embryos developing to the blastocyst stage in single medium was significantly greater compared to the sequential media (P < .005; n = number of embryos per media).
Nuclear Count
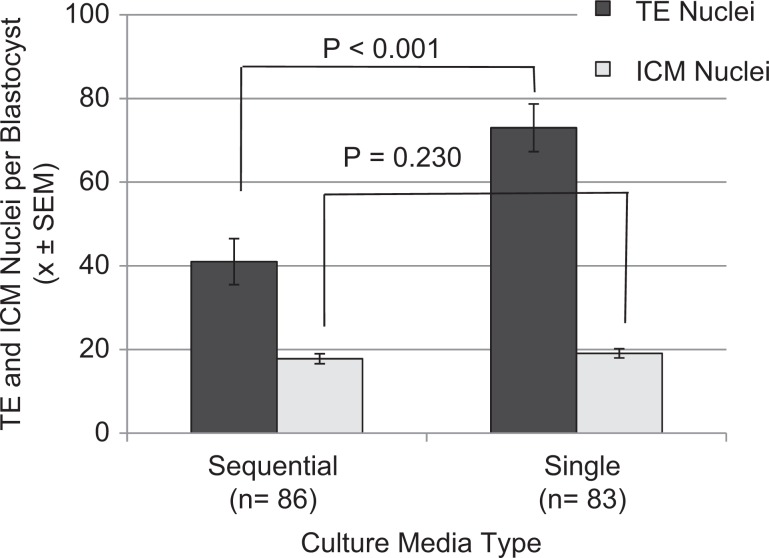
The single medium yielded a significantly higher number of trophectoderm (TE) nuclei per blastocyst versus the sequential media (mean difference = 33.24, CI 23.53-42.96, P < .0001; Figure 3), whereas no significant difference in the number of ICM nuclei per blastocyst was found between the two media types (OR = 1.37, CI -0.8858-3.6347, P=0.230; Figure 3). The ratio of presumptive TE to ICM nuclei per blastocyst was different between the two media, with single medium having greater odds having of more TE nuclei compared to sequential media (OR = 1.72, CI: 1.50-1.97, P < .0001).
Figure 3.
Number of trophoblast (TE) and inner cell mass (ICM) nuclei per blastocyst. More TE nuclei (P < .0001) but similar numbers of ICM nuclei (P < .230) were found in single versus sequential media. There was a significantly higher ratio of TE to ICM nuclei in single medium (P < .0001; n = total number of blastocysts per media type.)
Unhatched Versus Partially/Fully Hatched Blastocysts
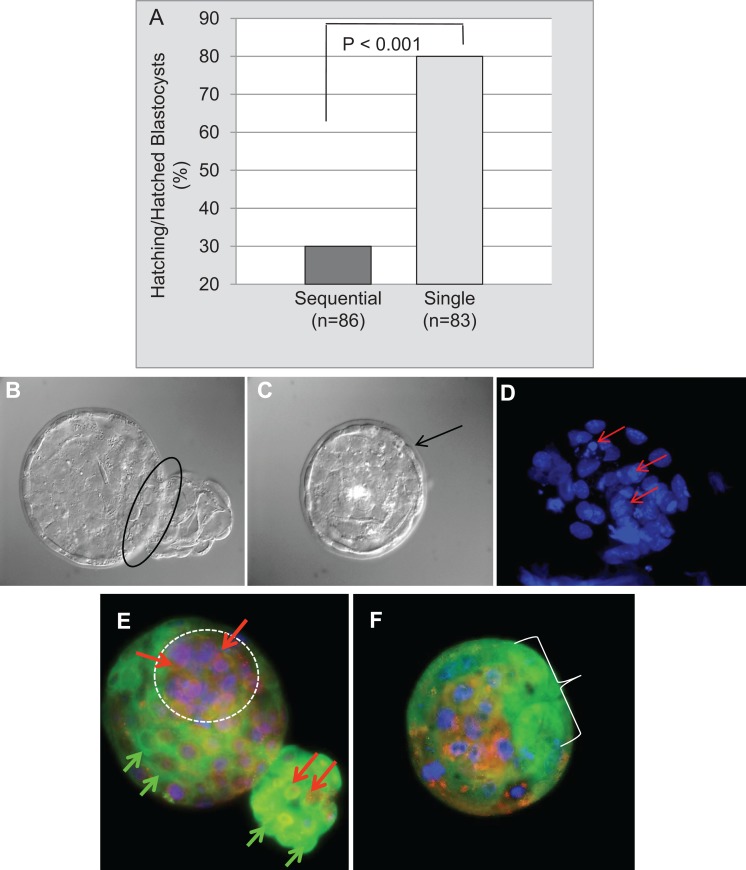
More blastocysts were hatched or hatching in the single medium; the odds of the single medium leading to a hatched blastocyst were almost 9 times greater than for the sequential media (OR = 8.90, CI 2.95-29.62, P = <.001; Figures 4A and 4B). Unhatched blastocysts cultured in the sequential media maintained an intact zona pellucida (Figure 4C) and various types of nuclear damage (Figure 4D). High-quality hatching blastocysts from single medium have a distinct ICM, where UCHL1 is localized in the cytoplasm and UCHL3 is localized in nuclei (Figure 4E). Low-quality, nonhatching blastocysts from sequential media have small nuclei with fragmentation, pyknosis, karyorrhexis, and/or chromosomal misalignment and poorly formed ICMs, where localization of UCHL1 and UCHL3 is diffuse and inappropriately dispersed throughout the cytoplasm and nuclei (Figure 4F).
Figure 4.
Blastocyst hatching, nuclear quality, and ubiquitin C-terminal hydrolase L1 (UCHL1)/ubiquitin C-terminal hydrolase L3 (UCHL3) localization on developmental day 5 per media type. A, More blastocysts hatched or were hatching in single medium versus sequential media (P < .001; n = total number of blastocysts per media.). B, Hatching blastocyst from single medium (black oval indicating trophectoderm emerging from zona pellucida). C, Unhatched blastocyst with intact zona pellucida (black arrow) from sequential media. D, Pyknosis (shrinking/condensation) and karyorrhexis (disintegration/granulation; red arrows) are forms of nuclear damage in this blastocyst cultured in sequential media. E, High-quality hatching blastocyst cultured in single medium has a distinctive clustering of cells in the inner cell mass (ICM) (white circle). UCHL1, green fluorescence (green arrows), localized in the cytoplasm and at the cell boarders and UCHL3, red fluorescence (red arrows), localized in nuclei. F, Lower quality nonhatching blastocyst cultured in sequential media with small nuclei of differing sizes, some undergoing pyknosis and karyorrhexis (see D), ill-defined trophectoderm cell borders lacking distinct UCHL1 staining (white bracket area) and a poorly formed ICM with indistinct UCHL3 red fluorescence dispersed throughout the cytoplasm compared to the hatching blastocysts with distinct cytoplasmic UCHL1 and nuclear UCHL3 patterns (see E; original magnification of 600×). The combination of nuclear damage and diffuse UCHL1 and UCHL3 staining was deemed indicative of reduced blastocyst developmental capacity.
Discussion
Using a murine embryo culture model, our data have shown single medium designed for human embryo culture leads to significantly more TE nuclei and a greater number of hatched blastocysts compared to sequential media. A higher proportion of TE to ICM nuclei was also observed in the single medium group. One factor contributing to these findings may be the embryo cleavage rate. As early as 1973, McLaren and Bowman reported that rapidly cleaving murine embryos had significantly higher birth and pregnancy rates when transferred from in vitro culture.24 Since that time, various studies have reported that in vitro rapidly dividing murine, porcine, bovine, and human embryos have more nuclei indicative of a higher total cell count and are of a higher quality compared to those that cleave slowly.25–28 In our study, more rapid embryo growth led to a higher blastocyst yield in single medium, concurrently associated with improved embryo quality.
We found that increased numbers of TE nuclei contributed to the significant difference in the total number of nuclei per blastocyst per media type. Increased numbers of TE nuclei in human and porcine blastocysts reportedly lead to a more complete and cohesive TE layer, enhancing the embryo’s ability to cavitate due to an increase in Na+/K+ ATPase activity and accumulation of aquaporin channels.29–31
Others reported that blastocysts with greater numbers of TE cells develop a more robust placenta in utero, improving sustenance of the fetus.26,27 It is possible that the increased number of hatching/hatched blastocysts in the present study was a result of a significantly higher number of TE nuclei in the single medium, leading to a higher rate of blastocyst expansion.
In contrast to single medium, the early stage medium of the sequential culture does not contain amino acids, glucose, and EDTA (Table 1). Differences in the single medium and sequential media components may thus contribute to the variation observed in embryo quality and development. Further, the osmolality of both components of the sequential media was 290 mOsm after equilibration, whereas the single medium was 270 mOsm. Osmolality pertains to the number of solutes present in the media. For example, if the sequential media contained higher levels of sodium chloride it may contribute to embryo dehydration and lower development potential. Components of the media used in this study (Table 1) cannot be assessed as they remain proprietary information.
Amino acids are osmolytes, regulators of intracellular pH, and reactive oxygen species scavengers.4–6 Culturing preimplantation cleavage stage murine embryos in media containing different amino acids improved developmental rates by nearly 20%, increasing blastocyst cell number and decreasing apoptosis.6 Glutamine, a known substrate of the glycine transporter GLY1 (expressed predominantly in precompacted embryos), is metabolized to glutamate, a nucleic acid precursor and α-ketoglutarate, an intermediate in the citric acid cycle.31 We propose that the sequential media lacking amino acids renders preimplantation embryos more susceptible to external stressors leading to suboptimal metabolism, a delay in blastomere cleavage rates, and increased embryo toxicity.
Glucose is another key component in preimplantation development. Early exposure of mouse embryos to glucose reportedly causes an up regulation of GLUT1, a glucose transporter present throughout all stages of development, along with monocarboxylate transporter 1, a pyruvate/lactate transporter.32 In vivo, precompacting embryos have a lower level of glucose up take in many species ranging from bovine to rodent, which contributes to the accumulation of mass needed for proper growth and development. We predict that the exposure of preimplantation embryos to lower glucose concentrations as in the single medium increases developmental potential and improves glucose uptake.
While the odds of embryos developing to ≥8C stage on d3 were more than 2 times greater in single medium than sequential media, and statistical significance was not reached (P = .06). Yet on d5, significantly more embryos reached the blastocyst stage in the single medium than the sequential media (P = .005). We cannot determine from these studies whether developmental differences did not exist until d5 or mechanisms altering embryo development were initiated prior to d3 but the anomalous embryonic phenotype did not manifest itself until after d3 of development. One might postulate that the sequential media disrupts proper blastocyst formation by damage from the first medium in the sequential pair. These observations add to a growing body of literature indicating ocular evaluation of morphological parameters alone is insufficient to determine embryo quality.
Ubiquitin is a small polypeptide “ubiquitously” found in eukaryotic cells that combines with other proteins to make them susceptible to degradation.33 The process of ubiquitination is a posttranslational modification signaling for lysosomal or proteasomal proteolysis that is regulated and reversed by DUBs. UCHL1 and UCHL3 are 2 of the DUBs regulating the oocyte/embryo ubiquitin–proteasome system by targeting and cleaving covalent ubiquitin–ubiquitin and ubiquitin–substrate protein.20,21 The aberrant, diffuse localization of UCHL1 and UCHL3 throughout the cells in poor quality embryos cultured in the sequential media may be used as markers of embryo quality and suggests dysregulation of ubiquitination and possible mechanistic functions for these enzymes in embryo development and/or degradation.
A variety of factors may contribute to the conflicting results of research on the quality of human embryo culture media. Methods used for evaluation and measurement of embryonic development in humans are divergent.17–20 This lack of consistency interferes with accurate interlaboratory comparisons. Albeit for several decades, murine embryo bioassays have been a standard of media quality control in many human IVF laboratories.34,35 It is possible that human embryo culture media may be more conducive to human than murine embryo development. Species specificity must be considered when interpreting the results of any embryo bioassay. However, when culture media are evaluated using human embryos, individual patient variation may also confound comparisons of media types, unless split cohorts of embryos per patient are tested. By using a single strain of murine embryos, we eliminated the potential for patient to patient variation, yet we does not exclude the fact that different strains of murine embryos might also develop differently. We acknowledge that our study used in vivo generated cryopreserved murine zygotes, whereas humans undergoing IVF would typically have generated zygotes in vitro. In vitro fertilization generated human zygotes might respond differently than in vivo fertilized murine zygotes to single versus sequential media. Lastly, the distinction between ICM and TE cells may have been improved with standard Oct 4 antibody screening, which was not performed.
Conclusions
In conclusion, the use of IVF for infertility treatment has become commonplace in today’s society. It is important to continue to improve and identify optimal culture media for human preimplantation embryo development in vitro. Considering the restrictions imposed by the United States Department of Health and Human Services on experimental research using human embryos, animal embryo bioassays remain a necessity in testing human embryo culture media.34 Here we report that single culture medium developed for human embryos supports superior murine embryo developmental competence as defined by an increased growth rate, greater numbers of TE cells, and greater numbers of hatching blastocysts. Distinct cytoplasmic UCHL1 and nuclear UCHL3 localization observed when culturing embryos in single medium versus diffuse staining of embryos cultured in sequential media correlates well with morphological assessment and developmental potential. Using the results from this study, we have begun investigating gene imprinting and expression and the ubiquitination process during embryo development from the 8C stage to the blastocyst stage to provide insights into the timing of initiation and mechanisms of the developmental differences.
Acknowledgments
The authors wish to thank the staff in Dr Sharpe-Timms’ laboratory in the Department of Obstetrics, Gynecology and Women’s Health Division of Perinatal Research, particularly Ms. Alexis Ruffolo. The studies performed in Kathy Sharpe-Timms’ laboratory were supported by the National Institute of Child Health and Human Development (R01 HD057445 to KST), the School of Medicine at the University of Missouri and Department of Obstetrics, Gynecology and Women’s Health. Studies performed in Peter Sutovsky’ s laboratory were supported by National Research Initiative Competitive Grant 2011-67015-20025 from the USDA National Institute of Food and Agriculture and by the Food for the 21st Century Program of the University of Missouri.
Footnotes
Author’s Note: Presented in part by Kathy Sharpe-Timms at the Annual Meeting of the American Society for Reproduction, October 18 to 22, 2014 in Honolulu, HI, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Han MS, Niwa K. Effects of BSA and fetal bovine serum in culture medium on develompent of rat embryos. J Reprod Dev. 2003;49(3):235–242. [DOI] [PubMed] [Google Scholar]
- 2. Naaktgeboren N. Quality control of culture media for in vitro fertilisation. Ann Biol Clin (Paris). 1987;45(3):368–372. [PubMed] [Google Scholar]
- 3. Oh SH, Miyoshi K, Funahashi H. Rat oocytes fertilized in modified rat 1-cell embryo culture medium containing a high sodium chloride concentration and bovine serum albumin maintain developmental ability to the blastocyst stage. Biol Reprod. 1998;59(4):884–889. [DOI] [PubMed] [Google Scholar]
- 4. Martin PM, Sutherland AE, Van Winkle LJ. Amino acid transport regulates blastocyst implantation. Biol Reprod. 2003;69(4):1101–1108. [DOI] [PubMed] [Google Scholar]
- 5. Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1(2):91–148. [DOI] [PubMed] [Google Scholar]
- 6. Lane M, Gardner DK. Amino acids and vitamins prevent culture-induced metabolic perturbations and associated loss of viability of mouse blastocysts. Hum Reprod. 1998;13(4):991–997. [DOI] [PubMed] [Google Scholar]
- 7. Almiñana C, Heath PR, Wilkinson S, et al. Early developing pig embryos mediate their own environment in the maternal tract. PLoS One. 2012;7(3):e33625 doi:10.1371/journal.pone.0033625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duranthon V, Watson AJ, Lonergan P. Preimplantation embryo programming: transcription, epigenetics, and culture environment. Reproduction. 2008;135(2):141–150. doi:10.1530/REP-07-0324. [DOI] [PubMed] [Google Scholar]
- 9. Whitten WK, Biggers JD. Complete development in vitro of the pre-implantation stages of the mouse in a simple chemically defined medium. J Reprod Fertil. 1968;17(2):399–401. [DOI] [PubMed] [Google Scholar]
- 10. Whitten WK. Culture of tubal mouse ova. Nature. 1956;177(4498):96. [DOI] [PubMed] [Google Scholar]
- 11. Whitten WK. Culture of tubal ova. Nature. 1957;179(4569):1081–1082. [DOI] [PubMed] [Google Scholar]
- 12. Biggers JD, McGinnis LK, Raffin M. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol Reprod. 2000;63(1):281–293. [DOI] [PubMed] [Google Scholar]
- 13. Miyoshi K, Funahashi H, Okuda K, Niwa K. Development of rat one-cell embryos in a chemically defined medium: effects of glucose, phosphate and osmolarity. J Reprod Fertil. 1994;100(1):21–26. [DOI] [PubMed] [Google Scholar]
- 14. Guérin P, Ménézo Y. Review: role of tubal environment in preimplantation embryogenesis: application to co-culture assays. Review. Zygote. 2011;19(1):47–54. doi:10.1017/S0967199410000092. [DOI] [PubMed] [Google Scholar]
- 15. Artini PG, Valentino V, Cela V, Cristello F, Vitè A, Genazzani AR. A randomized control comparison study of culture media (HTF versus P1) for human in vitro fertilization. Eur J Obstet Gynecol Reprod Biol. 2004;116(2):196–200. [DOI] [PubMed] [Google Scholar]
- 16. Sepúlveda S, Garcia J, Arriaga E, Diaz J, Noriega-Portella L, Noriega-Hoces L. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2009;91(5):1765–70. doi:10.1016/j.fertnstert.2008.02.169. [DOI] [PubMed] [Google Scholar]
- 17. Ciray HN, Aksoy T, Goktas C, Ozturk B, Bahceci M. Time-lapse evaluation of human embryo development in single versus sequential culture media--a sibling oocyte study. J Assist Reprod Genet. 2012;29(9):891–900. doi:10.1007/s10815-012-9818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paternot G, Debrock S, D’Hooghe TM, Spiessens C. Early embryo development in a sequential versus single medium: a randomized study. Reprod Biol Endocrinol. 2010;8:83 doi:10.1186/1477-7827-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling embryo study. Fertil Steril. 2009;92(5):1783–1786. doi:10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 20. Mtango NR, Sutovsky M, Vandevoort CA, Latham KE, Sutovsky P. Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation. J Cell Physiol. 2012;227(5):2022–20229. doi:10.1002/jcp.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mtango NR, Sutovsky M, Susor A, Zhong Z, Latham KE, Sutovsky P. Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development. J Cell Physiol. 2012;227(4):1592–1603. doi:10.1002/jcp.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi YJ, Manandhar G, Sutovsky M, et al. Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol Reprod. 2007;77(5):780–793. [DOI] [PubMed] [Google Scholar]
- 23. Sutovsky P. Visualization of sperm accessory structures in the mammalian spermatids, spermatozoa, and zygotes by immunofluorescence, confocal, and immunoelectron microscopy. Methods Mol Biol. 2004;253:59–77. [DOI] [PubMed] [Google Scholar]
- 24. McLaren A, Bowman P. Genetic effects on the timing of early development in the mouse. J Embryol Exp Morphol. 1973;30(2):491–498. [PubMed] [Google Scholar]
- 25. McLaren A, Bowman P, Lonergan P, O’Kearney-Flynn M, Boland MP. Effect of protein supplementation and presence of an antioxidant on the development of bovine zygotes in synthetic oviduct fluid medium under high or low oxygen tension. Theriogenology. 1999;51(8):1565–1576. [DOI] [PubMed] [Google Scholar]
- 26. Lonergan P, O’Kearney-Flynn M, Boland MP. Effect of protein supplementation and presence of an antioxidant on the development of bovine zygotes in synthetic oviduct fluid medium under high or low oxygen tension. Theriogenology. 1999;51(8):1565–1576. [DOI] [PubMed] [Google Scholar]
- 27. Luna M, Copperman AB, Duke M, Ezcurra D, Sandler B, Barritt J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (>or=10 cells), bringing into question current embryological dogma. Fertil Steril. 2008;89(2):358–363. [DOI] [PubMed] [Google Scholar]
- 28. Eckert J, Tao T, Niemann H. Ratio of inner cell mass and trophoblastic cells in blastocysts derived from porcine 4- and 8-cell embryos and isolated blastomeres cultured in vitro in the presence or absence of protein and human leukemia inhibitory factor. Biol Reprod. 1997;57(3):552–560. [DOI] [PubMed] [Google Scholar]
- 29. Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26(12):3289–3296. doi:10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 30. Houghton FD, Humpherson PG, Hawkhead JA, Hall CJ, Leese HJ, Na+ K+, ATPase activity in the human and bovine preimplantation embryo. Dev Biol. 2003;263(2):360–366. [DOI] [PubMed] [Google Scholar]
- 31. Kim SJ, Koo OJ, Kwon DK, et al. Replacement of glutamine with the dipeptide derivative alanyl-glutamine enhances in vitro maturation of porcine oocytes and development of embryos. Zygote. 2014;22(2):286–289. [DOI] [PubMed] [Google Scholar]
- 32. Purcell SH, Moley KH. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol Metab. 2009;20(10):483–489. doi:10.1016/j.tem.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pickart CM, Eddins MJ. “Ubiquitin: structures, functions, mechanisms”. Biochim Biophys Acta. 1695;(1–3):55–72. doi:10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 34. Gardner DK, Reed L, Linck D, Sheehan C, Lane M. Quality control in human in vitro fertilization. Semin Reprod Med. 2005;23(4):319–324. [DOI] [PubMed] [Google Scholar]
- 35. Vijayakumar R, Simoni J, Ndubisi B, DeLeon F, Heine W. Mouse embryo growth in different culture media: selection of a medium for quality control cross-testing of human in vitro fertilization conditions. Arch Androl. 1987;19(2):149–158. [DOI] [PubMed] [Google Scholar]