Abstract
Remodeling of the cervix occurs in advance of labor both at term and at preterm birth. Morphological characteristics associated with remodeling in rodents were assessed in cervix biopsies from women at term (39 weeks’ gestation) and preterm (<33 weeks’ gestation). Collagen I and III messenger RNA and hydroxyproline concentrations declined in cervix biopsies from women in labor at term and preterm compared to that in the cervix from nonlaboring women. Extracellular collagen was more degraded in sections of cervix from women at term, based on optical density of picrosirius red stain, versus that in biopsies from nonpregnant women. However, collagen structure was unchanged in the cervix from women at preterm labor versus the nonpregnant group. As an indication of inflammation, cell nuclei density was decreased in cervix biopsies from pregnant women irrespective of labor compared to the nonpregnant group. Moreover, CD68-stained macrophages increased to an equivalent extent in cervix subepithelium and stroma from groups in labor, both at term and preterm, as well as in women not in labor at term. Evidence for a similar inflammatory process in the remodeled cervix of women at term and preterm birth parallels results in rodent models. Thus, a conserved final common mechanism involving macrophages and inflammation may characterize the transition to a ripe cervix before birth at term and in advance of premature birth.
Keywords: parturition, ripening, preterm birth, inflammation
Introduction
Preterm birth (PTB) is a leading cause of neonatal mortality and morbidity.1 Although the mechanism that initiates labor both preterm and at term is multifactorial,2,3 remodeling of the cervix occurs throughout pregnancy and accelerates well before increases in prepartum uterine contractile activity.4 Early in pregnancy, up to 85% of the extracellular matrix (ECM) of the cervix is composed of densely structured collagen, predominantly type I,5 whereas smooth muscle may be 4% to 5% distally and 10% in the inner meatus.6–9 As the third trimester progresses, softening of the cervix is characterized by decreases in collagen content and more diffusely dissociated cross-linking of fibrils in the ECM.10 As remodeling continues, water content increases along with the ability to extract collagen from cervix tissue.11,12 Although evidence suggests that continued degradation of collagen, reduced cross-linking, and changes in proteoglycans enhance pliability of the cervix, less is known about the morphological characterization of this process leading up to term.13
Compared to studies of human cervix biopsy tissue, common structural remodeling features of the cervix have been reported in rodents.14–16 In addition to a shift in biomechanical properties to a more distensible and elastic phenotype,17 elongation of the cervix is associated with reduced density of cell nuclei (CN) as pregnancy nears term.14 Apoptosis of cells does not account for fewer cells/area of cervix prior to the onset of labor,18–20 rather ECM degradation, evidence for hypertrophy of cells, and lengthening of the cervix occur several days before labor and birth. Moreover, increases in macrophages (Mφs)/area characterize the progress of cervical remodeling, leading up to term,21,22 as well as in PTB induced by inflammation23 or after progesterone receptor (PR) antagonist treatment.24 Whether the density of CN declines in the cervix of pregnant women at term or during remodeling before PTB is not known.
Indeed, at term, more immune cells per microscopic field have been reported in women in labor compared to nonlaboring women.25 This observation is consistent with the concept that prepartum remodeling of the cervix reflects an inflammatory process.15,26–28 Elevated proinflammatory cytokines as well as increased activity by matrix metalloproteinases (messenger RNA [mRNA] and protein) also occur in the prepartum cervix even when infection is not apparent.27,28 Although an increased presence of neutrophils and Mφs is indicated in cervical biopsies from women at term in labor (TL) compared to those not in labor,25,29,30 little is known about the presence of leukocytes in the cervix of women at risk for PTB or in preterm labor (PTL).31 However, observations of stained immune cells in microscopic fields of view of human cervix biopsies do not account for morphological heterogeneity (vascularity, glands, etc) or subregional variability in size of cells that accompany the biopsy or remodeling process. Thus, whether the census of Mφs differs in the peripartum cervix of women at term or with PTL is not known. In the present study, we tested the hypothesis that reduced collagen-related gene expression, morphometric characteristics of the remodeling process, and increased presence of Mφs are advanced to the same extent in the cervix of women with PTL as occurs at term.
Methods
Patients and Cervix Biopsy
All procedures were approved by the local ethics committee of Karolinska Institute (Reference no 97-089 and 04-637/4), and each participant provided informed consent. Immediately following vaginal delivery or cesarean section, a biopsy was taken transvaginally from the anterior lip of the cervix at the 12-o’clock position with scissors and tweezers.27,32,33 Women with diagnoses of preeclampsia, diabetes, endometriosis, or systemic diseases related to infection were excluded from the study. None of the women presented with cervical dysplasia. There were no significant differences between these groups of women in maternal age, parity, previous PTBs, or previous cesarean sections.
Collagen RNA Expression and Hydroxyproline Analysis
A cervix biopsy was obtained from 6 women delivered preterm prior to the onset of labor by cesarean section (PTNL), 11 women delivered at term by cesarean section prior to the onset of labor (TNL), 13 with PTL, and 11 at TL. Biopsies were not available for nonpregnant women in this study. Patient demographics are in Table 1. Participants in the PTNL group presented with evidence of placental abruption or intrauterine growth retardation. In 4 of these patients, betamethasone was given from 6 hours to 6 days before the cervix biopsy was taken, 3 patients received a single dose, whereas 1 patient received this corticosteroid treatment once each week for 4 weeks. Patients in the PTL and TL groups were in active labor and demonstrated a ripe cervix, with dilatation more than 4 cm, and cervix biopsies were taken directly after vaginal delivery. In the PTL group, 3 patients received a dose of betamethasone at least 6 hours before the cervix biopsy was obtained. Women in the PTNL and TNL groups had an unripe cervix with a Bishop score of <5 and were delivered by cesarean section before the onset of labor. The indications for cesarean section in the TNL group were breech presentation, prior cesarean section, disproportion, or humanitarian considerations of maternal distress related to vaginal delivery as in our previous reports.
Table 1.
Patient Characteristics for mRNA and Hydroxyproline Analyses in Cervix Biopsies From Women With PTNL, TNL, PTL, and TL.
| PTNL | TNL | PTL | TL | |
|---|---|---|---|---|
| Number of patients | 6 | 11 | 13 | 11 |
| Age (range), years | 31 (27-35) | 30 (25-39) | 30 (18-39) | 30 (23-39) |
| Parity (range) | 1 (0-3) | 1 (0-2) | 1 (0-3) | 1 (0-3) |
| Prior preterm birth | 1 | 1 | 2 | 0 |
| Prior cesarean section | 0 | 6 | 3 | 2 |
| Weeks of gestation (range) | 30 (26-36) | 39 (37-40) | 33 (26-36) | 39 (37-42) |
| Days of gestation (range) | 209 (183-256) | 270 (263-284) | 233 (186-255) | 279 (259-294) |
Abbreviations: mRNA, messenger RNA; PTL, preterm spontaneous vaginal delivery; PTNL, preterm elective cesarean section; TL, term spontaneous vaginal delivery; TNL, term elective cesarean section.
Tissues were immediately frozen in liquid nitrogen and stored at −70°C for later analyses. Frozen tissue was cut into small slices on a block of dry ice and transferred to a prechilled (liquid nitrogen) capsule containing Teflon-coated tungsten balls. The capsule was kept in liquid nitrogen for 2 minutes and thereafter shaken in a dismembranation apparatus (Retsch KG, Haan, Germany) at full speed for 2 minutes. The procedure was repeated after intermediated freezing in liquid nitrogen until tissue was pulverized. Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions and stored at −70°C. For analysis of RNA concentration, 1 μg of total RNA was used for the reverse transcription reaction, and complementary DNA (cDNA) was stored at −70°C prior to use. The assay was completed using SuperScript RNase H− reverse transcriptase (Invitrogen) as described previously27 and was performed by Eppendorf BioPhotometer (Germany). All samples had an OD260–OD280 ratio >1.7.
Reverse transcription–polymerase chain reaction analysis was performed for 3 genes: housekeeping gene 28S, collagen type I, and collagen type III (Roche LightCycler, Germany). Sequences for primers are presented in Table 2. All the components for reactions were supplied in the LightCycler DNA Master SYBR Green I kit (Roche, Germany). Dilutions of the housekeeping gene were performed using 2 μL at 1:10, 1:100, 1:1000, whereas samples were diluted using 2 μL at 1:10 with sterile water. To individual LightCycler glass capillary tubes with respective dilutions, a reaction mixture was added that consisted of 2 μL “hot start” (FastStart Taq DNA polymerase, reaction buffer, deoxynucleotide mix, and SYBR Green I dye, Roche, Germany), 3.2 μL of MgCl2 (5 mmol/L), 0.8 μL (5 μmol/L) of 28S specific primer (sense and antisense), and adjusted volume of polymerase chain reaction (PCR) grade water (in this case 11.2 μL) to make the total reaction volume of 20 μL. Capillaries were sealed and centrifuged. Forty-five cycles of PCR (15 seconds of denaturation at 95°C, 10 seconds of annealing at 58°C, 20 seconds of extension at 72°C, and 5 seconds at 80°C) were run to ensure that the crossing point (cycle number) was attained even for the highest dilutions of standard cDNA. Fluorescence emission values were automatically monitored following each PCR cycle. Immediately after completion of cycling, the melting curve analysis (raising the sample temperature from 60°C to 98°C with constant monitoring of fluorescence emission) was carried out to control for the specificity of the PCR products obtained. For collagen I and III PCR, the mixture consisted of 2 μL hot start mix, 2.4 μL (4 mmol/L) of MgCl2, 0.4 μL (10 μmol/L) of collagen I or collagen III specific primer (sense and antisense), and adjusted volume of PCR grade water (12.8 μL). The annealing temperature for the PCR run was 59°C. Other conditions were the same as described previously. The size of PCR products was estimated by 1.5% agarose gel electrophoresis. The quantity of collagen I and collagen III mRNA was normalized to the 28S housekeeping gene.
Table 2.
Specifications of mRNA Species, Primer Sequences, PCR Annealing Temperature, and Product Size.
| mRNA | 5′ Primer | 3′ Primer | °C | Size (bp) |
|---|---|---|---|---|
| 28S | GTGCAGATCTTGGTGGTAGTAGC | AGAGCCAATCCTTATCCCGAAGTT | 58 | 552 |
| Collagen I | GTGAAAATGGAGCTCCTGGT | GAGCACCATTGGCACCTTTA | 59 | 310 |
| Collagen III | CTGAACTCAAGAGTGGAGAA | TCAGCCTTGAATTCACCTTC | 59 | 399 |
Abbreviations: bp, base pair; mRNA, messenger RNA; PCR, polymerase chain reaction.
For collagen determination, each frozen biopsy (<2 mm) was minced, delipidated with acetone (0.5 mL), and extracted 3 times each with 1 mL of 0.5 mol/L acetic acid and 0.5 mol/L of acetic acid containing pepsin (1 mg/mL). Extracts were centrifuged (1 hour, 20 000 g at 4°C), hydrolyzed in 2 mL 6 mol/L HCl at 100°C for 17 hours, lyophilized, and hydroxyproline analyzed as described previously.11,34
Collagen Stain, Mφ Immunohistochemistry, and Morphometric Analyses
Cervix biopsies were available for histological analyses from 4 groups of women, that is, nonpregnant women undergoing hysterectomy due to benign conditions such as myomas (NP, n = 4), at term delivered by cesarean section prior to the onset of labor (TNL, n = 7), preterm in labor (PTL, n = 9), and at TL (n = 9). Nonpregnant women had no history of cervix dysplasia and no hormone replacement therapy or other hormonal treatment. Women in the TNL group had an unripe cervix with a Bishop score of <5 and were delivered by cesarean section for reasons described previously. Women in the PTL and TL groups were in active labor, and cervical biopsy was taken directly after vaginal delivery. Patient information is provided in Table 3. Immediately after biopsy, tissue was rinsed in physiological saline, immersion fixed in 4% formaldehyde for up to 24 hours, and transferred to 70% ethanol until embedded in paraffin. Sections were cut at 10 μm and stained with picrosirius red (PSR) dye, and optical density (OD) was analyzed to assess collagen content and cross-link structure.35 Briefly, 8 nonoverlapping polarized black and white photomicrographs were taken of the center portion of 2 nonadjacent sections from each cervix biopsy (1.25 × 106 μm3, Spot 4 Mp Pursuit Camera PR1640 [Diagnostic Instruments, Inc., Sterling Heights, MI, USA] on a Zeiss Axio Imager microscope, Thornwood, NY, USA), and images were analyzed as described previously.24 Other sections were processed by immunohistochemistry with antigen retrieval (2-minute proteinase K; Dako North America, Carpinteria, California) to visualize CD68-stained Mφs (1:50 for 90 minutes; Dako and ABC kit; Vector Lab, Burlingame, California) and counterstained with methyl green to visualize CN. Sections lacking primary antibody incubation served as the negative control for each group of tissue stained. Following dehydration, sections were coverslipped and 8 photomicrographs taken in each of both the subepithelium and center subregions (1.25 × 106 μm3/subregion). To ensure the most representative analysis of each photomicrograph, care was taken to avoid areas containing experimental artifacts such as tissue tearing, as well as blood vessels. Images were analyzed using the cell counter plugin as part of ImageJ.35 Macrophages were counted if they had a dark brown 3,3′-diaminobenzidine (DAB) reaction product in a well-defined area associated with methyl green-stained CN.
Table 3.
Patient Characteristics for Immunohistochemistry Study of Cervix Biopsy Sections From Women Who Were NP, TNL, PTL, and TL.
| NP | TNL | PTL | TL | |
|---|---|---|---|---|
| Number of patients | 3 | 7 | 9 | 9 |
| Age (range), years | 44 (37-49) | 33 (26-39) | 34 (29-38) | 30 (23-40) |
| Parity (range) | 0 | 1 (0-2) | 0 (0-1) | 0 (0-1) |
| Prior preterm birth | 0 | 0 | 1 | 0 |
| Prior cesarean section | 0 | 1 | 0 | 0 |
| Weeks of gestation (range) | 0 | 39 (37-39) | 33 (26-36) | 39 (38-41) |
| Days of gestation (range) | 0 | 271 (264-278) | 233 (187-251) | 280 (271-287) |
Abbreviations: NP, nonpregnant; PTL, women at preterm spontaneous vaginal delivery; TL, women at term with spontaneous vaginal delivery; TNL, women who delivered via cesarean section prior to labor.
Statistical Analysis
Data for collagen (OD of PSR stain birefringence) and Mφ numbers/area were normalized to CN/area to account for hypertrophy, edema, and morphological variability among sections and individuals as in previous studies of rodents.23,24,35,36 Areas of analysis in sections from each cervix biopsy were empirically determined to accurately reflect the average OD and cell densities. Data were analyzed by analysis of variance (ANOVA) with Dunnett test for individual comparisons compared to NP controls or by the Kruskal-Wallis test with Dunn for multiple comparisons (Prizm 5.01; GraphPad Software Inc, California). For both tests, P < .05 was considered significant.
Results
Collagen mRNA Expression and Hydroxyproline Concentrations
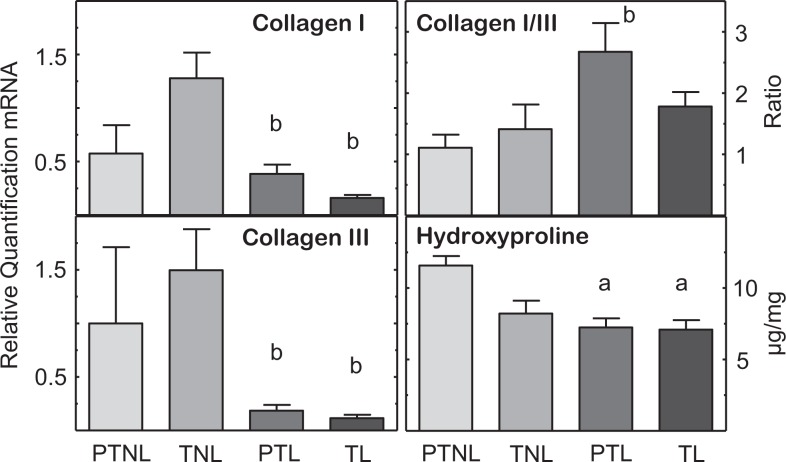
Collagen I and III mRNAs were not different in cervix biopsies from women not in labor, whether preterm or at term (Figure 1; P > .05, PTNL = TNL). However, women in the PTL and TL groups had reduced levels of collagen I and III compared to the TNL group (P < .05). Across groups, only the ratio of collagen I–III was increased for the PTL versus PTNL group. The diminished mRNA for collagen I and III in groups of women in labor was accompanied by reduced soluble collagen as indicated by lower hydroxyproline concentrations in the PTL and TL groups compared to that in cervix biopsies from PTNL women.
Figure 1.
Expression of messenger RNAs (mRNAs) encoding collagen I and III, ratio of collagen I–III mRNA, and hydroxyproline concentrations (μg/mg wet weight, mean ± standard error [SE]) in cervix biopsies from pregnant women who were preterm not in labor (PNTL, n = 5-6), at term not in labor (TNL, n = 8-11), preterm in labor (PTL, n = 13), or at term in labor (TL, n = 8-11). Cervix biopsies were obtained at cesarean section (details in Methods). P < .05, Kruskal-Wallis with Dunn test for individual comparisons versus aPTNL or bTNL group.
Collagen Content and Structure
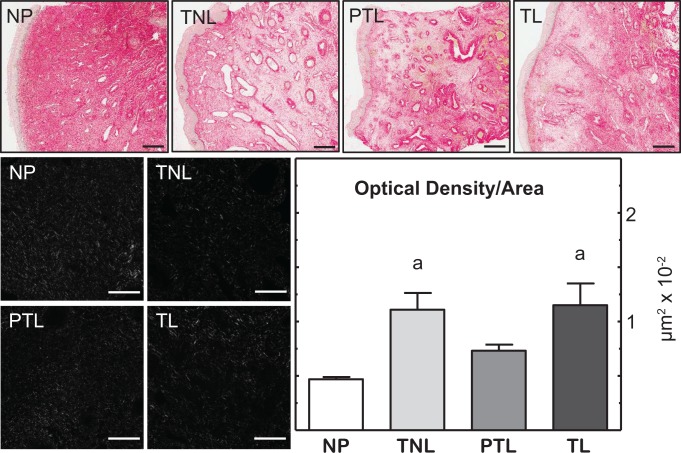
Size and morphology of cervix biopsies varied across individuals and groups. The heterogeneous morphology in sections was most evident when stained for collagen (Figure 2 shows photomicrographs of PSR-stained cervix and transformed photomicrographs analyzed for OD). Blood vessels and glands were abundant in biopsies but smaller in cervix from NP compared to pregnant women. Methods established in rodents indicate an inverse relationship between OD of birefringence of polarized light and collagen content and structure. With this approach to analyze cervix biopsy sections, OD increased in the center stromal region from pregnant versus NP women. The OD of cervix sections from TNL and TL women was greater than in the NP group (P < .05, ANOVA). No statistical differences in collagen content and structure were evident in sections of cervix among other groups of women whether at term or preterm, irrespective of labor.
Figure 2.
Picrosirius red-stained sections of cervix biopsies from women who were nonpregnant (NP, patient N8), term not in labor (TNL, patient N17), preterm labor (PTL, patient 301), and term labor (TL, patient 202). Biopsy morphology typically consisted of luminal epithelium (far left) with blood vessels and gland throughout center subregion. Red intensity reflects birefringence of collagen content and fiber structure. Scale bars = 500 μm. Black and white photomicrographs of polarized light from center stroma subregion reflect birefringence of extracellular collagen from each group. Variations from black to light reflect sparse to dense collagen and cross-linked structure. Analysis of photomicrographs (8-9 photomicrographs = 3.76 × 106 μm3) with NIH ImageJ with the Rodbard transformation quantified the inverse relationship between optical density (OD) and extracellular collagen content and structure (see Methods for details of analyses). Scale bars = 50 μm. a P < .05, Kruskal-Wallis with Dunn test versus the NP group.
Density of CN and Mφs
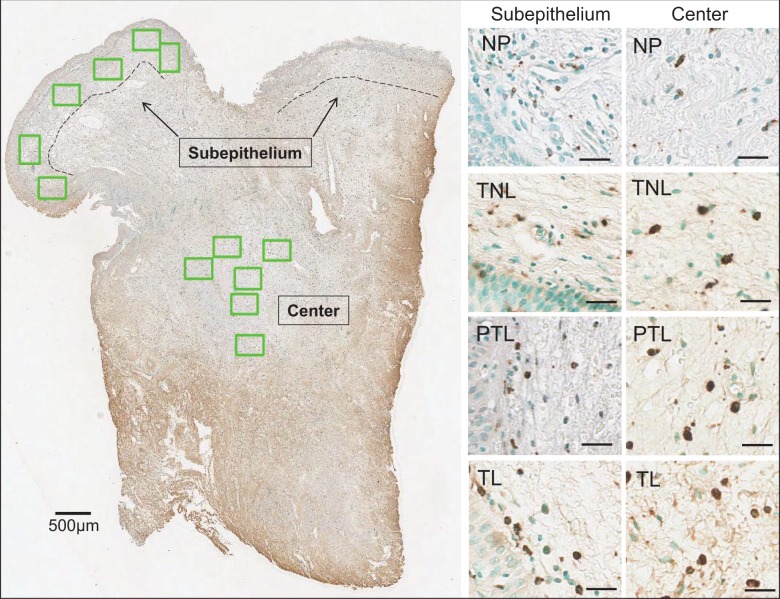
Although morphology of biopsies varied, common characteristics included luminal epithelial cell layers, a subepithelium that transitioned into a more vascularized center area with glands, and areas along cut edges with extravascular erythrocytes (Figure 3, left panel). Photomicrographs were taken within the subepithelium and center areas to count methyl green-counterstained CN and CD68 Mφs with dark brown DAB deposits associated with CN (Figure 3, right panels). Macrophages in biopsy tissue from NP women were sparse in subepithelial and center regions, but in pregnant women, Mφs were more abundant throughout the tissue and present within blood vessels.
Figure 3.
Left, Scan of cervix biopsy section from a woman in the PTL groups (patient 321). Subepithelium and center subregions were delineated by the dashed line (500 μm from inner luminal cell layer). At or near the cut edges of the biopsy, few cell nuclei and abundance of extracellular red blood cells precluded analyses for density of cells. In other regions, nonoverlapping photomicrographs were taken (6 boxes green highlight = 7.5 × 106 μm3) to count cell nuclei and macrophages (Mφs) as described in Methods. Right, Photomicrographs (20×) of subepithelium and center subregions from scans of sections from a cervix biopsy from women in the nonpregnant (NP), term not in labor (TNL), preterm labor (PTL), and term labor (TL) groups. Methyl green-counterstained cell nuclei and brown 3,3′-diaminobenzidine (DAB)-stained CD68 Mφs from each subregion luminal cells appear in bottom left corners of subepithelium photomicrographs. Scale bars = 25 μm.
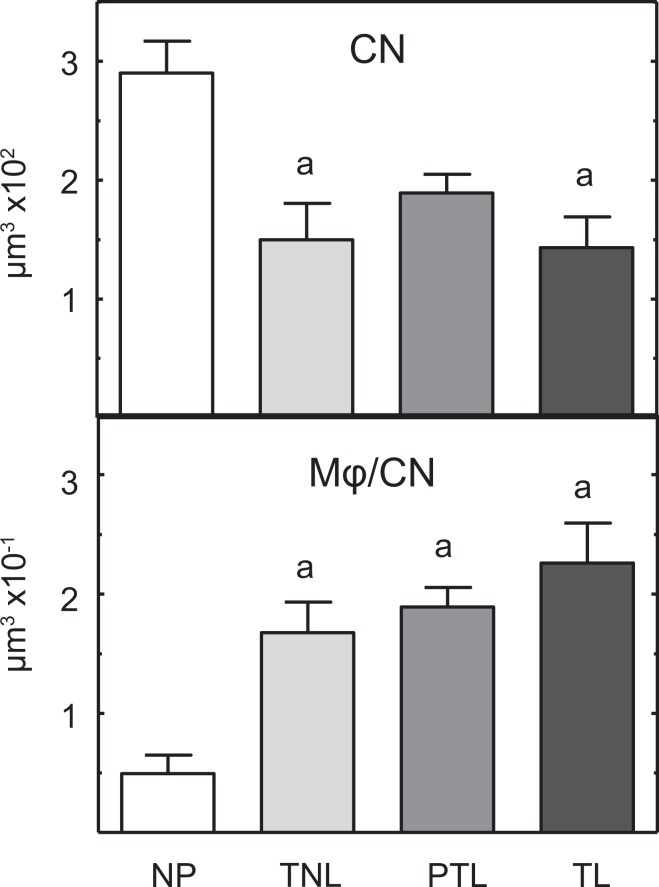
In nonoverlapping photomicrographs that were taken to exclude cell-sparse areas and specific structures (glands, blood vessels, and tissue tears), CN density decreased with pregnancy, significantly so for the TNL and TL groups compared to that in NP women (Figure 4, top; P < .05, ANOVA). No difference was evident in CN/area among TNL, PTL, or TL groups. These same differences were found in subepithelium and center regions (data not shown). Although the present data set was normally distributed (P > .05, Levine test), further analyses of the mean, not normalized to CN, as well as the median interquartile range/high-power field, as done by Osman et al25, indicated no significant differences among TNL, PTL, and TL groups in the study (P > .1 ANOVA and Kruskal-Wallis).
Figure 4.
Mean (±standard error [SE]) density of cell nuclei (CN) per volume and density of macrophages (Mφs) normalized to CN in cervix biopsies from women who were nonpregnant (NP; n = 3), term not in labor (TNL; n = 7), preterm labor (PTL; n = 9), and term in labor (TL; n = 9). a P < .05, One-way analysis of variance (ANOVA) with Dunnett test versus the NP group.
Using the same approach as in rodent studies to account for variations in cellular morphology across sections and individuals (detailed in Methods), the density of Mφs increased in the cervix of women in PTL, as well as at term irrespective of labor (Figure 4, bottom; P < .05 NP < TNL = PTL = TL). Analysis of the median interquartile range of Mφ numbers/high-power field was also not significant among TNL, TL, and PTL groups (median/range: 24/17-24, 27/19-48, and 39/28-55, respectively; P > .05 Kruskal-Wallis with Dunn test). Macrophages density was not different among pregnant women in PTL and at term whether or not in labor. Analysis of subregions indicated these same differences among groups for Mφ density in the center region, but variability precluded significance in the subepithelium of cervix.
Discussion
The present study determined that remodeling processes in the peripartum cervix of women share specific characteristics associated with restructuring of the cervix in rodents as pregnancy nears term. Analyses indicate for the first time that the CN density of the cervix, that is, CN/area, declined in groups at term irrespective of labor, compared to that in nonpregnant women. Fewer cells in a comparable cervix biopsy area in term groups compared to nonpregnant women are suggestive of increase size of cells in the stroma or expansion of the ECM. Evidence for ECM collagen degradation in laboring tissues at term is indicated by reduced hydroxyproline and decreased collagen types I and III mRNA in the present study. These findings are consistent with reports of enhanced collagenases and metalloproteinases, both mRNA and protein, in the peripartum cervix,11,33,37 rather than apoptosis reducing CN density of cells in the prepartum cervix.18,19 In rodents, similar remodeling characteristics are found about 3 to 5 days before the day of birth in the cervix from pregnant mice and rats24,35 along with reduced tensile strength.38 However, in contrast to rodents where structural changes result in a lengthening of the prepartum cervix, the cervix in pregnant women does not appear to lengthen near term,39 Ekman-Ordeberg personal observations. Evidence for expansion of the cervix with reduced collagen content and structure raises the possibility that lateral stretching and thinning increase the width (diameter) of the preterm cervix in women to maintain as closed an internal Os as possible during the transition from remodeling to ripening. With respect to morphological observation by Leppert et al,40 the present findings suggest that CN density may reflect processes associated with ECM degradation and expansion of cells in the cervix of women as in other species at term.
To further characterize the remodeling process, use of picrosirius dye indicated that collagen content and structure is reduced in cervix biopsies from women at term. Increased OD of PSR-stained birefringence is consistent with the decline in hydroxyproline.13 This conclusion corresponds with previous reports in rats at term that temporally correlate extracellular remodeling characteristics with biomechanical assessment of stretch parameters by the prepartum cervix.41–43 However, biopsies from the PTL group had reduced hydroxyproline, as well as collagen I and III concentrations, but no statistical difference was observed in either OD of PSR-stained sections or CN density compared to parameters in tissue from controls (PTNL or NP, respectively). This finding raises the possibility that collagen content had declined, but ECM structure had yet to more fully degrade as at term. Assessment of ECM characteristics in a biopsy may not reflect the heterogeneous morphology that is characteristic of the prepartum cervix.42,43 Separate analyses of subepithelium and center regions within each biopsy in the present study and in a previous report30 did not indicate difference in remodeling characteristics within each group. This concern is not necessarily relevant to findings for the rodent cervix, where analyses surveyed a relatively large area within longitudinal sections from the Os to the transition zone before the uterine body. Thus, with respect to the availability of biopsy material from a diverse patient population, further evidence is needed to support the possibility that structural remodeling of the cervix at term is advanced with PTB.
More compelling are the findings that inflammatory processes in the cervix are enhanced at term and with PTB because of increased presence of Mφs. Higher concentrations of matrix metalloproteinases and inflammatory cytokines in the prepartum cervix could result from increased activity by more resident Mφs both during preterm and term labor.27,28,32,33 Findings in multiple strains of mice and rats similarly and consistently indicate an increase in Mφ density relative to CN/area in the cervix before the day of birth compared to earlier in pregnancy or in nonpregnant controls.24,35 An accurate estimation of cell density/area in cervix in this model depends upon evaluation of a sufficient area/section empirically determined from the number of photomicrographs needed to plateau the moving average of cell counts/field of view. Enumerating Mφs in subepithelium and stroma, while excluding those in luminal epithelium, vessels, and crypts, also minimizes errors in perception of resident cell abundance that could result from variable distribution of stained immune cells in photomicrographs taken within and among sections from the same individual. This approach addresses technical concerns associated with the lack of immunohistochemical stain for Mφs within the cervical stroma from prepartum mice.44,45 Moreover, methodological concern about a flow cytometry study that reported a small population of Mφs remained unchanged in the cervix as pregnancy neared term46 was later addressed by an another flow study22 which found that (1) perfusion was needed to accurately assess resident Mφs, an approach that corrected for vascular expansion and increased presence of systemic immune cells in the prepartum cervix and (2) gate settings using a live cell marker and flow analysis of another dispersed tissue, that is, spleen, were necessary to accurately estimate the increase in resident Mφs in the prepartum cervix versus earlier in pregnancy or in nonpregnant controls. The confirmation of immunohistochemical findings by flow cytometry adds to the consensus of other reports25,30 that Mφs are more abundant in the prepartum cervix compared to earlier in pregnancy across multiple mammalian species.
Although counts were as accurate as possible in the present study, the density of Mφs was equivalently elevated in the TNL and TL groups (whether calculated as mean or median quartile range), a finding that did not confirm the result of Osman et al that Mφs were less abundant in nonlaboring versus laboring women.25 This difference may reflect methodological differences between studies, that is, fixation (paraformaldehyde vs formalin), normalized versus not normalized data sets, areas counted in selected photomicrographs, biopsy morphology, and unspecified duration of labor. The broader perspective of these and other studies is to support an increased presence of Mφs in the late remodeled ripened cervix of women at term and with PTL compared to the less or unremodeled state earlier in pregnancy and in nonpregnant women. This consensus directly corresponds to findings in rodents at term and preterm remodeling induced by inflammation or PR antagonist treatment.14,24,35,36,47 Collectively, the results support the hypothesis that ripening of the cervix in women at term reflects an inflammatory process that may be advanced with PTB.
Understanding the structural characteristics associated with the remodeling processes in the cervix of women during pregnancy has several important clinical implications. Evidence for breakdown of collagen and the ECM as part of a physiological inflammatory mechanism raises the possibility that several variables might be separately monitored to assess the progress of remodeling with pregnancy.48 Minimally invasive imaging approaches might prove useful for the assessment of cervix cell density, structure, and inflammation. In addition to the potential diagnostic value, characteristics associated with remodeling may help morphologically define features that result in a short cervix, the current standard to predict risk for PTB in women.49 Findings also suggest that evaluation of width or diameter of the cervix might be a useful parameter to include in the ultrasound evaluation of cervix competency. Finally, novel therapeutic possibilities might result from interventions that regulate inflammatory processes both preterm or postterm with premature or delayed remodeling of the cervix, respectively. Thus, the novel contribution of the present finding in women is that characteristics associated with remodeling of the cervix at term reflect enhanced inflammatory processes that appear to be conserved across several mammalian species and are possibly advanced with PTB.
Acknowledgements
The authors thank Anne Heuerman, Melisa Custer, Abigail Dobyns, and John Hough for technical support to process tissue, analysis data, and graph. The authors are grateful for the help of Professor Donald R. Chase, Professor of Pathology and Human Anatomy, California Tumor Tissue Registry at Loma Linda University.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by NIH HD054931 and the Loma Linda University Department of Pediatrics. Imaging was performed in the LLUSM Advanced Imaging and Microscopy Core with support of NSF Grant MRI-DBI 0923559 and the Loma Linda University School of Medicine.
References
- 1. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):r525–r545. [DOI] [PubMed] [Google Scholar]
- 4. Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25(1):69–79. [DOI] [PubMed] [Google Scholar]
- 5. Iwahashi M, Muragaki Y, Ooshima A, Umesaki N. Decreased type I collagen expression in human uterine cervix during pregnancy. J Clin Endocrinol Metab. 2003;88(5):2231–2235. [DOI] [PubMed] [Google Scholar]
- 6. Danforth DN. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am J Obstet Gynecol. 1947;53(4):541–560. [DOI] [PubMed] [Google Scholar]
- 7. Schwalm H, Dubrauszky V. The structure of the musculature of the human uterus—muscles and connective tissue. Am J Obstet Gynecol. 1966;94(3):391–404. [DOI] [PubMed] [Google Scholar]
- 8. Rorie DK, Newton M. Histologic and chemical studies of the smooth muscle in the human cervix and uterus. Am J Obstet Gynecol. 1967;99(4):466–469. [DOI] [PubMed] [Google Scholar]
- 9. Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn. 2013;33(8):737–741. [DOI] [PubMed] [Google Scholar]
- 10. Onizawa S. Studies on changes in the uterine cervix during pregnancy [in Japanese]. Nihon Sanka Fujinka Gakkai Zasshi. 1987;39(4):633–640. [PubMed] [Google Scholar]
- 11. Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147(6):662–666. [DOI] [PubMed] [Google Scholar]
- 12. Granstrom L, Ekman G, Ulmsten U, Malmstrom A. Changes in the connective tissue of corpus and cervix uteri during ripening and labour in term pregnancy. Br J Obstet Gynaecol. 1989;96(10):1198–1202. [DOI] [PubMed] [Google Scholar]
- 13. Ekman G, Malmstrom A, Uldbjerg N, Ulmsten U. Cervical collagen: an important regulator of cervical function in term labor. Obstet Gynecol. 1986;67(5):633–636. [PubMed] [Google Scholar]
- 14. Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61(4):879–883. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol. 2013;97(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida K, Jiang H, Kim M, et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One. 2014;9(11): e112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garfield RE, Saade G, Buhimschi C, et al. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4(5):673–695. [DOI] [PubMed] [Google Scholar]
- 18. Kemp B, Rath W, Winkler M, Reineke T, Beier HM, von Rango U. Is cervical dilatation during parturition at term associated with apoptosis?. J Perinat Med. 2005;33(2):137–143. [DOI] [PubMed] [Google Scholar]
- 19. Allaire AD, D’Andrea N, Truong P, McMahon MJ, Lessey BA. Cervical stroma apoptosis in pregnancy. Obstet Gynecol. 2001;97(3):399–403. [DOI] [PubMed] [Google Scholar]
- 20. Leppert PC. Proliferation and apoptosis of fibroblasts and smooth muscle cells in rat uterine cervix throughout gestation and the effect of the antiprogesterone onapristone. Am J Obstet Gynecol. 1998;178(4):713–725. [DOI] [PubMed] [Google Scholar]
- 21. Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10(6):323–338. [DOI] [PubMed] [Google Scholar]
- 22. Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87(5):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009;16(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One. 2013;8(12): e81340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. [DOI] [PubMed] [Google Scholar]
- 26. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215. [DOI] [PubMed] [Google Scholar]
- 27. Dubicke A, Fransson E, Centini G, et al. Pro-inflammatory and anti-inflammatory cytokines in human preterm and term cervical ripening. J Reprod Immunol. 2010;84(2):176–185. [DOI] [PubMed] [Google Scholar]
- 28. Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005;3:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sennstrom MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6(4):375–381. [DOI] [PubMed] [Google Scholar]
- 30. Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66(2):161–173. [DOI] [PubMed] [Google Scholar]
- 31. Whitworth MK, Pafilis I, Vince G, Quenby S. Cervical leukocyte sub-populations in idiopathic preterm labour. J Reprod Immunol. 2007;75(1):48–55. [DOI] [PubMed] [Google Scholar]
- 32. Dubicke A, Andersson P, Fransson E, et al. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010;84(1):86–94. [DOI] [PubMed] [Google Scholar]
- 33. Dubicke A, Akerud A, Sennstrom M, et al. Different secretion patterns of matrix metalloproteinases and IL-8 and effect of corticotropin-releasing hormone in preterm and term cervical fibroblasts. Mol Hum Reprod. 2008;14(11):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–273. [DOI] [PubMed] [Google Scholar]
- 35. Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85(3):498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nold C, Maubert M, Anton L, Yellon S, Elovitz MA. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol. 2013;208(3):223.e221–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norman M, Ekman G, Malmstrom A. Changed proteoglycan metabolism in human cervix immediately after spontaneous vaginal delivery. Obstet Gynecol. 1993;81(2):217–223. [PubMed] [Google Scholar]
- 38. Feltovich H, Ji H, Janowski JW, Delance NC, Moran CC, Chien EK. Effects of selective and nonselective PGE2 receptor agonists on cervical tensile strength and collagen organization and microstructure in the pregnant rat at term. Am J Obstet Gynecol. 2005;192(3):753–760. [DOI] [PubMed] [Google Scholar]
- 39. Meath AJ, Ramsey PS, Mulholland TA, Rosenquist RG, Lesnick T, Ramin KD. Comparative longitudinal study of cervical length and induced shortening changes among singleton, twin, and triplet pregnancies. Am J Obstet Gynecol. 2005;192(5):1410–1415. [DOI] [PubMed] [Google Scholar]
- 40. Leppert PC, Cerreta JM, Mandl I. Orientation of elastic fibers in the human cervix. Am J Obstet Gynecol. 1986;155(1):219–224. [DOI] [PubMed] [Google Scholar]
- 41. Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Changes in cervical resistance and collagen fluorescence during gestation in rats. J Perinatal Med. 1999;27(3):188–194. [DOI] [PubMed] [Google Scholar]
- 42. Danforth DN. The morphology of the human cervix. Clin Obstet Gynecol. 1983;26(1):7–13. [DOI] [PubMed] [Google Scholar]
- 43. Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Munoz del Rio A, Hall TJ. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet Gynecol. 2014;43(4):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6(11):e26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathology. 2011;179(2):838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86(2):39. [DOI] [PubMed] [Google Scholar]
- 48. Menon R, Torloni MR, Voltolini C, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci. 2011;18(11):1046–1070. [DOI] [PubMed] [Google Scholar]
- 49. Iams JD, Grobman WA, Lozitska A, et al. Adherence to criteria for transvaginal ultrasound imaging and measurement of cervical length. Am J Obstet Gynecol. 2013;209(4):365.e361–e365. [DOI] [PMC free article] [PubMed] [Google Scholar]