Abstract
To understand the changes in the structural integrity of fetal membranes during intrauterine inflammation, we evaluated the time course of expression and localization of damage-associated molecular patterns (DAMPs) and injury/remodeling in collagen and vascular smooth muscle. Time-mated ewes received intra-amniotic (IA) saline or IA lipopolysaccharide (LPS) for 5 hours to 15 days prior to a preterm delivery at 125 ± 2 days (n = 5-7 animals/group). The DAMP high mobility group box 1 (HMGB1) protein assessed by Western blot was induced within 24 hours after IA LPS in the fetal membranes, and HMGB1 expression was localized to amnion epithelium, chorion vascular endothelium, and infiltrating inflammatory cells by immunohistology. Markers of vascular injury, intercellular adhesion molecule 1, and tissue plasminogen activator messenger RNA (mRNA) expression increased 5 to 12 hours after IA LPS in the chorioamnion indicating vascular injury. Chorion vascular remodeling with increased chorion arteriolar smooth muscle actin expression by morphometric analyses of immunohistology was noted 15 days after IA LPS. Collagen expression was nonhomogeneous by histochemical staining, and there was a trend toward decreased mRNA expression of collagen subunit COL5A1 after IA LPS.
Conclusions:
Intrauterine inflammation induced early increases in HMGB1 in the chorioamnion with a concomitant vascular injury followed by chorion arteriolar hypertrophy. There was nonhomogeneous collagen expression in the chorioamnion. These results have implications for understanding the pathogenesis of IA inflammation-induced preterm rupture of membranes.
Keywords: intrauterine infection, preterm labor, alarmins, HMGB1, cytokines
Introduction
Acute histologic chorioamnionitis is an inflammatory lesion of the placenta frequently observed in preterm birth. The presence of acute chorioamnionitis is associated with an increased risk of fetal organ injury.1-9 One of the common associations of chorioamnionitis is prelabor rupture of membrane (PROM).10 Prelabor rupture of membrane can predispose to chorioamnionitis by permitting migration of microorganisms from lower genital tract to upper genital tract.11-18 Microorganisms in the amniotic fluid or sterile inflammation, an intra-amniotic (IA) inflammation without any detectable microorganisms, are associated with PROM.19,20 A major class of inflammatory products inducing sterile inflammation are termed as “damage associated molecular patterns (DAMPs)” or “alarmins.”19,21,22 Amniotic fluid concentrations of some DAMPs high mobility group box 1 (HMGB1) and heat shock protein 70 (HSP70) are increased in chorioamnionitis.19,23 Further, Romero et al19 showed that amniotic fluid HMGB1 concentrations are increased in patients with IA inflammation compared to those without it in both preterm delivery and preterm PROM. In addition, HMGB1 was immunolocalized to the cytoplasm of amnion epithelium cells, myofibroblasts and macrophages of chorioamniotic membranes, and infiltrating neutrophils in patients with histologic chorioamnionitis. However, the temporal relationships between DAMPs, chorioamnionitis, and the compromised integrity of the fetal membranes are not fully understood.
The DAMPs are of great current interest because of their role in inflammation-associated organ injury in ischemia–reperfusion injury, cardiovascular diseases, and diabetes mellitus.21 The protein HMGB1 and its receptor-advanced glycation end products (RAGEs) are prototypic DAMPs. The HMGB1 is normally in the nucleus, but it can induce cytokine-like responses via controlled exocytosis from cells or by its release during necrosis.24-26 The DAMP HMGB1 is particularly interesting for perinatal medicine since amniotic fluid HMGB1 levels increase in patients with preterm labor/preterm PROM with IA infection/inflammation.19 Although the association of increased amniotic fluid HMGB1 with preterm delivery is strong, the source of HMGB1 is not known in cases of IA inflammation.27 The amniotic fluid concentrations of other DAMPs, soluble RAGE, were increased in patients with clinical chorioamnionitis at term, and HSP70 concentrations were increased in patients with histological chorioamnionitis.23,28
We use preterm sheep given IA injections of proinflammatory agonists to cause chorioamnionitis and multiple fetal organ injury responses similar to those reported in humans.2,29 We hypothesized that IA injection of lipopolysaccharide (LPS) would increase DAMPs in the chorioamnion and compromise integrity of the fetal membranes. Preterm fetal sheep were delivered at 7 time points ranging from 5 hours to 15 days after an IA injection of LPS. Proinflammatory cytokines and DAMPs were measured, and markers of structural integrity of the fetal membranes were assessed after surgical delivery of the preterm fetus.
Material and Methods
Animals
The Animal Care and Use Committees of the Cincinnati Children’s Hospital Medical Center and University of Western Australia approved the study protocol. All procedures involving animals were performed at The University of Western Australia (Perth, Australia). Time-mated Merino ewes with singleton fetuses were randomly assigned (50 ewes) to groups for exposure to either 10 mg of IA LPS from Escherichia coli (O55: B5; Sigma Aldrich, St Louis, Missouri) diluted in 2 mL of sterile saline (n = 45) or 2 mL of sterile saline as control (n = 5) at varying intervals prior to delivery. The 10 mg dose of LPS given by IA route was based on our earlier dose finding experiments and reliably causes chorioamnionitis, IA inflammation, and fetal inflammatory responses.30-32 The LPS was given by IA injection with ultrasound guidance30 at 5 hours, 12 hours, 24 hours, 2 days, 4 days, 8 days, or 15 days prior to preterm delivery (5-7 animals per time point) to study the time course of inflammation, injury, and repair phenomenon in the chorionamnion. The control animals (n = 5) are a composite of IA saline injection at different time points prior to delivery. All the animals (controls and experimental) were delivered surgically at 125 ± 2 days of gestation. Thus, the experiment controlled for gestation-related changes in the fetal membranes between controls and experimental groups but not the length of exposure to the IA injection. Ewes were euthanized with 100 mg/kg of intravenous pentobarbital with rapid surgical delivery of the fetus.30 Chorioamnion membrane was quickly dissected and snap frozen for messenger RNA (mRNA) analysis. Chorioamnion was also fixed with 10% buffered formalin for histology and immunohistochemistry.30 The inflammatory response of the fetal thymus and spleen and DAMP response in the fetal lung were reported previously for this series of animals.33-35
Messenger RNA Quantification
Total RNA was isolated from chorioamnion after homogenization with TRIzol (Invitrogen, Carlsbad, California) as previously described.30 Reverse transcription was performed using Verso complementary DNA (cDNA) kit (Thermo Scientific, Waltham, Massachusetts) to produce single-stranded cDNA. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was carried out in a StepOnePlus Real Time PCR system (Life Technologies, Grand Island, New York) with cycling conditions of a 2-minute incubation at 50°C, followed by one 10-minute incubation at 95°C, followed by 40 cycles of alternating temperatures of 95°C for 15 seconds and 60°C for 1 minute. At the end of each cycle, normalized fluorescence was recorded by the StepOnePlus Real Time PCR system (Life Technologies). Quantitative RT-PCRs were performed with sheep-specific TaqMan gene expression primers (Life Technologies) for the proinflammatory cytokines/chemokines (interleukin 1α [IL-1α], IL-1β, tumor necrosis factor α [TNF-α], membrane cofactor protein 1 [MCP-1], IL-6, and IL-8), DAMPs (HMGB1 and RAGE), vascular injury (tissue plasminogen activator [t-PA], plasminogen activator inhibitor 1 [PAI1], endothelial nitric oxide synthase (NOSIII), vascular endothelial growth factor A [VegFA], vascular endothelial growth factor receptor 1 [VegFR1], VegFR2, intercellular adhesion molecule [ICAM]), and basement membrane subunits (COL1A1, COL3A1, COL4A1, COL5A1, COL6A1, and fibronectin 1). The genes were chosen as representative for each family of proteins.2,21,22,36-38 The mRNA expression of each gene was normalized to the mRNA of the ribosomal protein 18s as an internal standard. Final data were expressed as fold increase over the control value.
Immunohistochemistry
Immunohistology was performed with sections from formalin-fixed chorioamnion.39 Paraffin blocks were deparaffinized and rehydrated before microwave-assisted antigen retrieval in citric acid buffer at pH 6.0. Endogenous peroxidase activity was blocked with methyl alcohol/hydrogen peroxide. Sections were incubated overnight at 4°C with the primary antibody diluted in 2% serum in phosphate-buffered saline to block nonspecific reactions. We used the following primary antibodies: lactoferrin (Abcam, Cambridge, Massachusetts, cat # ab15811, dilution 1:50), HMGB1 (R&D Systems, Minneapolis, Minneapolis, cat # MAB1690, dilution 1:50), RAGE (AbD Serotec, Oxford, United Kingdom, cat # AHP594, dilution 1:1000), HSP70 (Biogenex, San Ramon, California, cat # MU289-UC, dilution 1:50), caspase 3 (LSBio, Seattle, Washington, cat # LS-B2845, dilution 1:50), high mobility group nucleosome binding domain 1 (HMGN1; Novus Biologicals, Littleton, Colorado, cat # NBP1-78102, dilution 1:50), VEGFR2 (Cell Signaling, Danvers, Massachusetts, cat # 2479L, dilution 1:300), and monoclonal anti-α smooth muscle actin (SMA; Sigma, cat # A5228, dilution 1:10 000). Sections were then washed and incubated with the appropriate species-specific secondary antibody diluted 1:200 in 2% serum for 2 hours at room temperature. After further washing, antigen:antibody complexes were visualized using a Vectastain ABC peroxidase kit (Vector Laboratories Inc, Burlingame, California). Antigen detection was enhanced with nickel-diaminobenzidine, followed by incubation with Tris-cobalt. Slides were counterstained with Nuclear Fast Red for photomicroscopy. To further characterize changes in the extracellular matrix and vascular structures of the chorionamnion, Masson trichrome staining for detection of collagen deposition and Weigert method of staining for elastic fibers was performed.36
Western Blot Protein Analysis
For Western blot measurements with the chorioamnion, tissue was homogenized in 50 mmol/L Tris-HCl buffer (pH7.5), 1 mmol/L ethylene glycol tetraacetic acid, 1 mmol/L EDTA containing protease inhibitor cocktail (Sigma-Aldrich) and 1 mmol/L phenylmethylsulfonyl fluoride (Sigma-Aldrich).36 Homogenates were centrifuged, and protein concentrations were determined by BCA protein assay reagent kit (Pierce, Rockford, Illinois). Twenty micrograms of protein were denatured, electrophoresed on 10% to 20% Tris-glycine gel (Invitrogen), and transferred to 0.25-μm nitrocellulose membrane (Bio-Rad). Membranes were blocked with 5% Blotto (Santa Cruz Biotechnology, Inc, Dallas, TX, USA) in Tris-buffered saline with Tween and then incubated overnight with HMGB1 (MAB1690; R&D systems) at 1:1000 or mouse monoclonal anti-α SMA (A5228; Sigma) at 1:2500 or antismooth muscle myosin heavy chain 11 (ab53219) at 1:1000 or anti β-actin (ab6276; Abcam) at 1:10 000 or anti-α tubulin (ab18251; Abcam) at 1:5000 antibody dilutions. Appropriate secondary antibodies conjugated with horseradish peroxidase were added at 1:5000 dilutions. Immunoreactivity was detected by using SuperSignal West Dura chemiluminescent reagents (Pierce). The blots were then imaged on ImageQuant LAS4000 Imager (GE Healthcare Biosciences, Pittsburgh, PA, USA) and quantified (Multigauge 2.0 software, GE Healthcare Biosciences, Pittsburgh, PA, USA).
Vascular Morphometry
Muscularis media of chorion arterioles was demarcated using α-SMA immunostaining. Five transversely sectioned chorion blood vessels per lamb with approximately similar diameter were chosen in a random and blinded manner (N = 4 lambs/group), and images were digitally acquired and the α-SMA staining was quantitated using a digital color threshold (Metamorph version 7.7 software; Molecular Devices, Sunnyvale, California). Morphometric measurements of chorion blood vessel smooth muscle thickness were analyzed as area of α-SMA expressed as percentage of total vessel diameter.
Statistical Analysis
Values are expressed as median or means ± standard deviations. All analyses were performed using the software GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California). The mRNA expression levels were compared using 2-tailed nonparametric t tests (Mann-Whitney) or 2-way Kruskal Wallis nonparametric analysis of variance (ANOVA), as appropriate to compare each experimental group to the control group. Values of P < .05 were considered significant.
Results
Animals
All animals exposed to IA LPS or saline were alive at delivery, and there were no differences in birth weight or cord pH values among the 5 to 7 animals per group.
Proinflammatory Cytokines
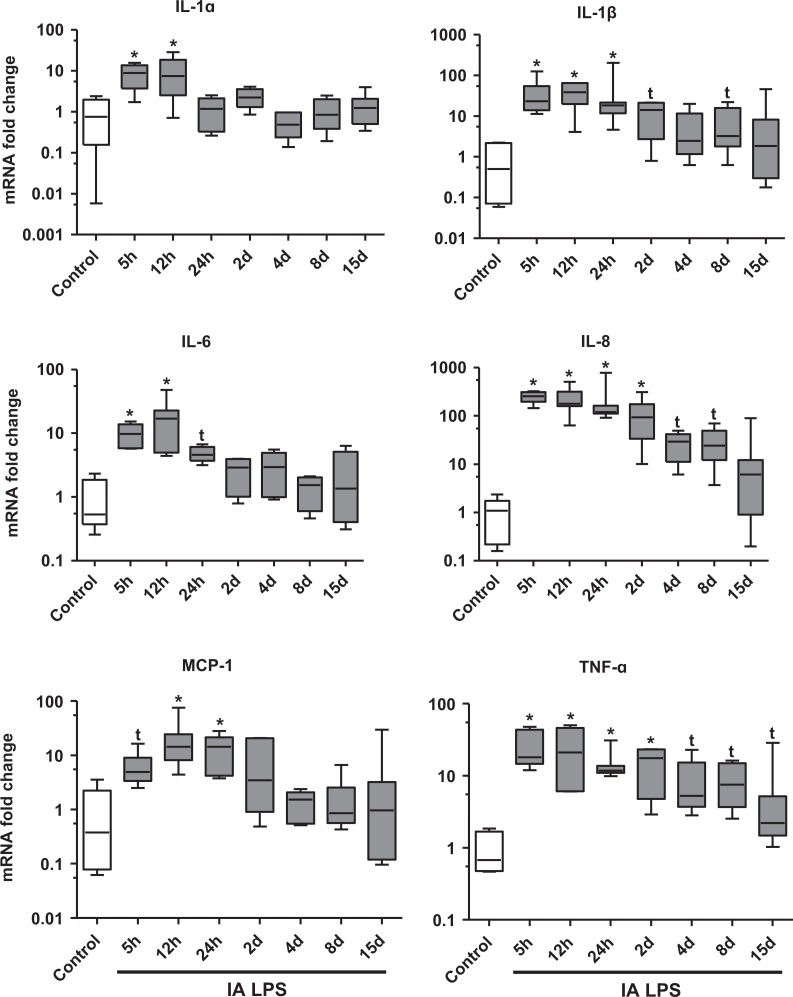
To characterize the expression of proinflammatory cytokines in the chorioamnion after IA LPS, we measured the mRNAs of IL-1α, IL-1β, IL-6, IL-8, MCP-1, and TNF-α. The cytokine expression increased from 5 hours to 2 days after IA LPS (Figure 1). In addition, there were trends for increases in mRNAs of IL-1β, IL-8, and TNF-α for 8 to 15 days after IA LPS.
Figure 1.
Quantitative mRNA expression of cytokines increased in fetal membranes after IA LPS exposure: Data shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median. The IL-1α, IL-1β, IL-6, IL-8, monocyte chemoattactant protein 1 (MCP-1), and tumor necrosis factor α (TNF-α) mRNA expression increased in the chorioamnion relative to saline controls from 5 to 48 hours after IA LPS exposure. Note the log scale (*P < .05 compared vs control by Kruskal-Wallis nonparametric analysis of variance [ANOVA] with post hoc Dunn test and by nonparametric t test, t P < .05 compared vs control by nonparametric t test only). IA indicates intra-amniotic; IL, interleukin; LPS, lipopolysaccharide; mRNA, messenger RNA.
Expression of DAMPs
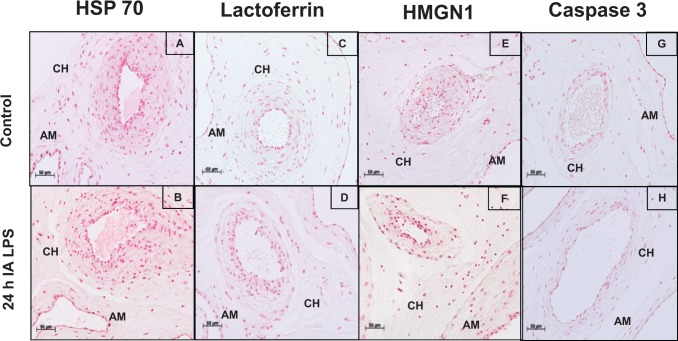
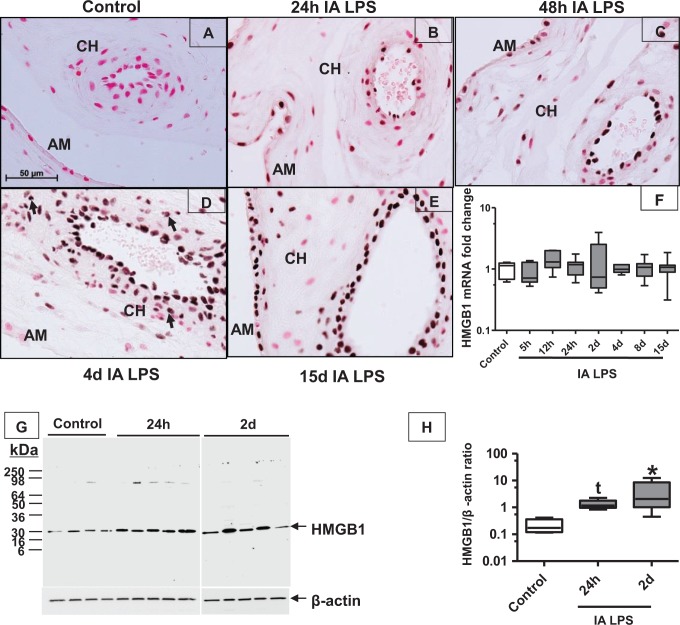
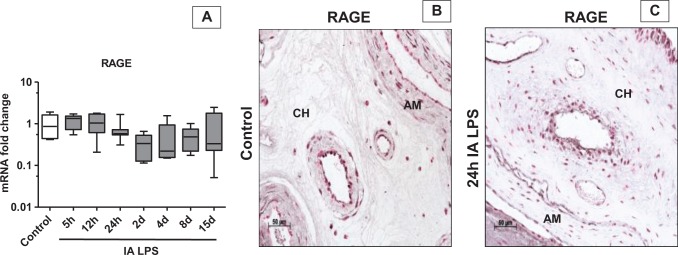
To characterize apoptosis and the expression of DAMPs in the chorioamnion, we performed immunohistochemistry for HSP 70, lactoferrin, HMGN1, and caspase 3 in the chorioamnion at different time points after IA LPS. There was little to no expression of these DAMPs, and the pattern of expression was similar from 5 hours to 15 days after IA LPS (Figure 2 shows representative immunohistochemistry at 24 hours after IA LPS vs controls). Importantly, no differences between controls and experimental animals were detected. In contrast, compared to control (Figure 3A), immunostaining of HMGB1 increased in the amnion epithelial cells at 5 hours (data not shown) after IA LPS and persisted for 15 days (Figure 3B-E). In addition, from 24 hours to 4 days after IA LPS (Figure 3B-D), HMGB1 expression was also increased in the vascular endothelium and in the infiltrating inflammatory cells (see arrow in Figure 3D). Increased HMGB1 expression in the amnion and vascular endothelium persisted for 15 days after IA LPS (Figure 3E). Consistent with the immunohistology, HMGB1 levels were significantly increased by Western blot by ∼7-fold at 24 hours and by 12-fold at 2 days after IA LPS exposure compared to control animals (Figure 3G and H). However, the mRNA expression of HMGB1 did not increase after LPS exposure (Figure 3F), suggesting that the HMGB1 induction was mediated by posttranscriptional events. Changes in the expression of RAGE (receptor for HMGB1) have been implicated in sterile inflammation.28 However, the mRNA expression and immunostaining of RAGE did not increase after exposure to IA LPS, which signals through Toll-like receptor 4 (TLR4; Figure 4A-C).
Figure 2.
Expression of several DAMPs in fetal membranes did not increase after IA LPS exposure: representative photomicrographs (20× objective) of the immunohistochemistry for HSP 70 showed staining in the amnion (AM) and chorion (CH) in control (A) and 24 hours after IA LPS exposure (B); lactoferrin in control (C) and 24 hours after IA LPS exposure (D); high mobility group nucleosome binding domain 1 (HMGN1) in control (E) and 24 hours after IA LPS exposure (F); and caspase 3 in control (G) and 24 hours after IA LPS exposure (H). No differences were observed in the expression of these DAMPs. DAMP indicates damage-associated molecular pattern; IA, intra-amniotic; LPS, lipopolysaccharide.
Figure 3.
The HMGB1 expression increased in fetal membranes after IA LPS exposure: photomicrographs (40× objective) of the immunohistochemistry for HMGB1 showed no baseline staining of the chorioamnion in the saline control (A) with increased expression amnion (AM) and chorion (CH) at 24 hours after intra-amniotic LPS (B), with further increases throughout the time course to 15 days after IA LPS (C-E). Data for (F) and (H) are shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median. The mRNA quantitation of HMGB1 by reverse transcription-polymerase chain reaction (RT-PCR) showing no change in expression after IA LPS (F). Western blot of HMGB1 showed increased expression at 24 hours to 2 days after intra-amniotic LPS (G-H; *P < .05 compared vs control by Kruskal-Wallis nonparametric analysis of variance [ANOVA] with post hoc Dunn test and by nonparametric t test, t P < .05 compared vs control by nonparametric t test only). HMGB1 indicates high mobility group box 1; IA, intra-amniotic; LPS, lipopolysaccharide.
Figure 4.
Expression of RAGE did not increase in fetal membranes after IA LPS exposure: data for (A) are shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median. Expression of RAGE messenger RNA (mRNA) by quantitative reverse transcription-polymerase chain reaction (RT-PCR) showed no change throughout the time course (A). Representative photomicrographs (20× objective) of immunohistochemistry for RAGE showed staining of the amnion (AM) and chorion (CH) in control (B) and 24 hours after IA LPS exposure (C) but there were no differences in the LPS-exposed animals compared to control. IA indicates intra-amniotic; LPS, lipopolysaccharide; RAGE, receptor-advanced glycation end product
Expression of Proteins in Blood Vessel
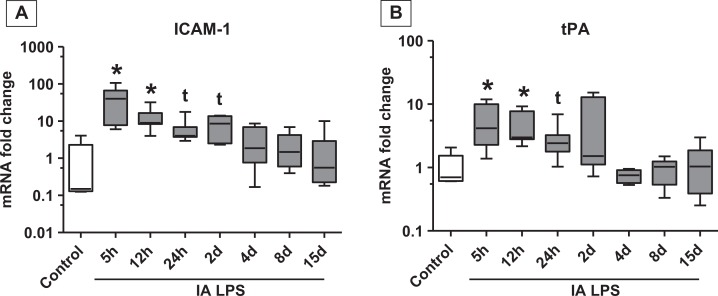
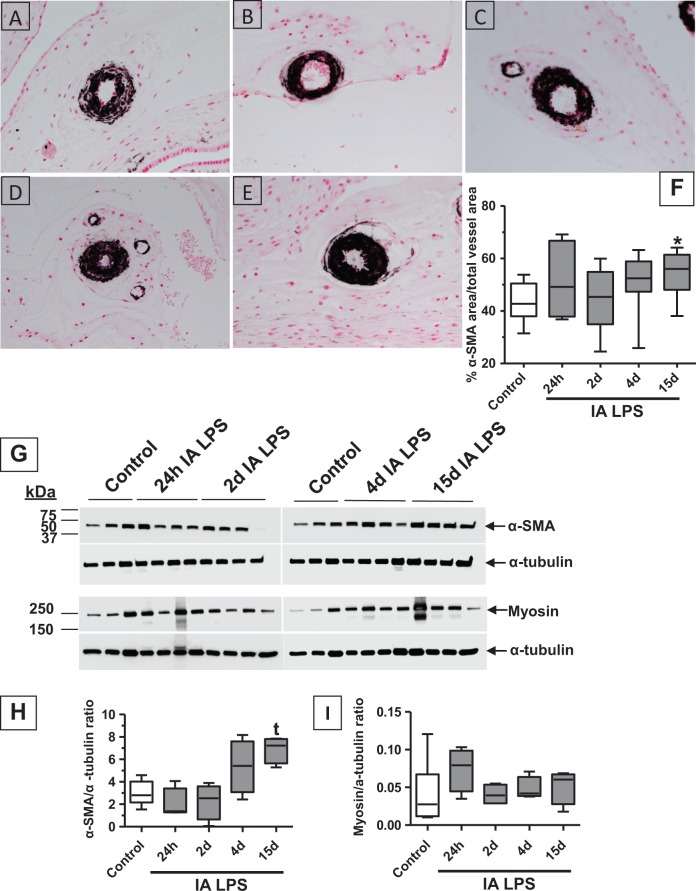
Since HMGB1 was expressed in the vascular endothelium, we characterized the vascular injury response in the chorion. The mRNA expression of ICAM-1 and tissue plasminogen activator (t-PA), known markers of vascular injury,40-42 was increased in the chorioamnion after IA LPS (Figure 5A and B). The mRNAs of the angiogenesis-related genes (VEGFA, VEGFR1, VEGFR2, PAI1, and NOSIII) did not change after IA LPS (Table 1). Vascular remodeling was evaluated by immunohistochemical staining of α-SMA, a marker of arterial smooth muscle hypertrophy.36 There was a qualitative increase in deposition of α-SMA from 24 hours up to 15 days after LPS exposure in the muscularis media of the chorion blood vessels (Figure 6A-E). Chorion arteriolar smooth muscle changes in α-SMA staining were quantitated morphometrically. The smooth muscle area increased from 43% in controls to 54%, 15 days after IA LPS treatment (Figure 6F). Western blot analysis of fetal membrane homogenates showed a 2-fold increase in α-SMA protein 15 days after IA LPS treatment compared to controls, albeit the significance was demonstrated by only a t test and not by ANOVA (Figure 6 G-H). However, no changes in the expression of myosin heavy chain were seen after IA LPS (Figure 6G and I). No changes in the elastic fiber staining were noted (data not shown).
Figure 5.
Expression of markers of vascular injury in fetal membranes increased after intra-amniotic (IA) lipopolysaccharide (LPS) exposure: data shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) showed increases in intercellular adhesion molecule 1 (ICAM-1) mRNA (A) and tissue plasminogen activator (t-PA) mRNA (B; *P < .05 compared vs control by Kruskal-Wallis nonparametric analysis of variance [ANOVA] with post hoc Dunn test and by nonparametric t test, t P < .05 compared vs control by nonparametric t test only). mRNA indicates messenger RNA.
Table 1.
Expression of Vascular-Related mRNAs in Chorioamnion.
| Gene | Control | 5 Hours LPS | 12 Hours LPS | 24 Hours LPS | 2 Days LPS | 4 Days LPS | 8 Days LPS | 15 Days LPS |
|---|---|---|---|---|---|---|---|---|
| VegFA | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.3 ± 0.5 | 1.5 ± 0.9 | 2.0 ± 1.2 | 1.2 ± 1.2 | 1.2 ± 0.4 | 1.6 ± 1.4 |
| VegFR1 | 1.0 ± 0.8 | 1.45 ± 0.9 | 1.2 ± 1.0 | 1.7 ± 1.4 | 2.8 ± 3.8 | 2.1 ± 2.4 | 1.5 ± 0.9 | 2.1 ± 1.4 |
| VegFR2 | 1.0 ± 0.8 | 0.9 ± 0.9 | 1.2 ± 0.7 | 1.1 ± 1.2 | 1.1 ± 0.9 | 0.9 ± 0.4 | 0.6 ± 0.3 | 1.6 ± 1.0 |
| NOSIII | 1.0 ± 0.8 | 1.15 ± 1.9 | 2.6 ± 4.1 | 0.5 ± 0.5 | 1.1 ± 0.6 | 1.8 ± 1.8 | 0.9 ± 0.5 | 1.1 ± 1.3 |
| PAI1 | 1.0 ± 0.7 | 2.4 ± 1.6 | 2.1 ± 2.0 | 0.6 ± 0.5 | 0.6 ± 0.3 | 0.4 ± 0.3 | 0.5 ± 0.3 | 0.9 ± 1.2 |
Abbreviations: mRNA, messenger RNA; NOSIII, nitric oxide synthase III; LPS, lipopolysaccharide; PAI1, plasminogen activator inhibitor 1; VegFA, vascular endothelial growth factor A; VegFR1, vascular endothelial growth factor receptor 1; VegFR2, vascular endothelial growth factor receptor 2.
Figure 6.
Chorion arteriolar smooth muscle hypertrophy after IA LPS exposure: representative photomicrographs (40× objective) of immunohistochemical staining for α-SMA showed expression in the vascular smooth muscle of chorion arteriole in control (A), with increased expression 15 days after IA LPS (E). Data for (F), (H), and (I) are shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median. Morphometric analysis of α-SMA staining in the vessel showed increased area of α-SMA expression relative to total vessel area 15 days after IA LPS exposure indicating smooth muscle hypertrophy (F). Western blot analysis of α-SMA showing a trend toward increased expression at 15 days after IA LPS (G-H) supporting the observations of smooth muscle hypertrophy. Western blot analysis of smooth muscle myosin heavy chain showed no significant changes in expression after IA LPS (G and I; *P < .05 compared vs control by Kruskal-Wallis nonparametric analysis of variance [ANOVA] with post hoc Dunn test and by nonparametric t test, t P < .05 compared vs control by nonparametric t test only). IA indicates intra-amniotic; LPS, lipopolysaccharide; SMA, smooth muscle actin.
Structural Protein Expression in the Chorioamnion
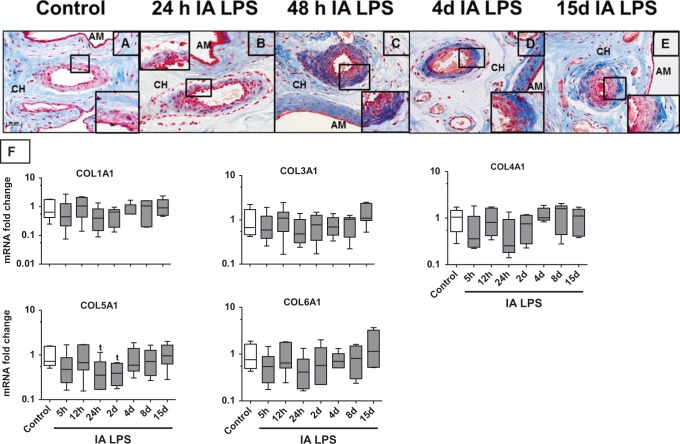
Collagen is a major extracellular matrix protein of the chorionamnion.37 Collagen fibrils stained uniformly throughout the chorioamnion of control animals (Figure 7A). Twenty-four hours after IA LPS, collagen staining decreased throughout the chorion but selective increased collagen expression was demonstrated in the blood vessel adventitia of the chorion (Figure 7B). This staining intensity increased around the vasculature in the chorion for up to 15 days (Figure 7B-E) after IA LPS (note the higher magnification of the blood vessels in the insets of each frame). There also appeared to be a corresponding decrease in stain throughout the matrix of the chorion. Furthermore, collagen staining was heterogenous instead of the homogenous distribution in the control fetal membranes (amnion and chorion). To quantify the changes in collagen, mRNAs of different collagen subtypes were measured in the chorioamnion (Figure 7F). There was a trend toward decreased expression of COL5A1 at 24 hours and 2 days after IA LPS with similar nonsignificant trends for the other collagen subtypes. The mRNA of fibronectin 1 did not change after IA LPS (data not shown).
Figure 7.
Heterogeneous expression of collagen in the fetal membranes after IA LPS exposure: representative photomicrographs (40× objective) of Masson trichrome collagen staining showed staining in the chorion (CH) and amnion (AM). Note the uniform staining of collagen in control (A). Decreased collagen staining in the chorion and increasing stain in the adventitia of the vasculature 24 hours after IA LPS (B); 48 hours after IA LPS (C). Continued decreased staining in the chorion with disorganized patchy appearance and increased stain in the amnion and around the vasculature 4 days after IA LPS (D). Recovery but patchy collagen staining in the chorion and continued increased stain around the vascular structures 15 days after IA LPS (E); quantitative reverse transcription-polymerase chain reaction (RT-PCR) for COL1A1-COL6A1 messenger RNAs (mRNAs) in the fetal membranes showed a trend toward decreased expression for COL5A1 after IA LPS exposure (F). Data for (F) are shown as box (25th-75th percentile) and whisker (10th-90th percentile) plot with horizontal line in each box depicting the median (t P < .05 compared vs control by nonparametric t test only and not by analysis of variance [ANOVA]). IA indicates intra-amniotic; LPS, lipopolysaccharide
Discussion
In a fetal sheep model of LPS-induced IA inflammation induced by LPS, HMGB1 expression was induced in the chorionamnion within 5 hours and persisted for 15 days. Notably, of the DAMPs studied, HMGB1 was the only DAMP whose expression was induced in this model of IA inflammation. We report the novel finding that in addition to the expression in the amnion epithelium, HMGB1 also increased in the vascular endothelium of the chorion. Importantly, the early vascular endothelial expression of HMGB1 was associated with indicators of vascular injury and a late remodeling of the chorion arterioles. Another novel finding was the dysmorphic or heterogeneous expression of collagen, a major matrix structural protein of the fetal membranes. Our results demonstrate parallel change in proinflammatory mediators, HMGB1, and indicators of compromised structural integrity of fetal membranes. These results offer insights into how IA inflammation could progress to the pathogenesis of PROM.
HMGB1 was proposed to be a late mediator of sepsis-induced lethality in mice.43 However, in our study, the expression of HMGB1 in the chorioamnion increased early in parallel with the proinflammatory cytokines. It should be noted that in our study, HMGB1 mRNA did not change although the protein level increased in the chorioamnion exposed to LPS suggesting a posttranscriptional control of HMGB1 synthesis by LPS. Indeed, the expression of HMGB1 is reported to be regulated at a translational level via miR-1192 and HuR in muscle cells upon injury.44 HMGB1 can signal through the receptor RAGE, TLR2, and TLR4.26 We previously reported that IA LPS increased TLR2 and TLR4 mRNA expression in the chorioamnion of preterm fetal sheep,45 similar to women with chorioamnionitis.46 The expression of RAGE did not change after IA LPS in this study. Interestingly, in the same animals, HMGB1 was ubiquitously expressed in the fetal lungs but the expression did not increase after IA LPS.34 Another DAMP, HSP70 expression increased in the fetal lung but did not change in the chorioamnion of preterm fetal sheep.34 These results demonstrated that there were different DAMP responses to IA LPS in different fetal organs in the fetal sheep. Amniotic fluid HSP70 was reported to be increased in women with acute chorioamnionitis.23 Thus different tissues/compartments can have different HSP70 responses to acute chorioamnionitis. The persistent increase in the expression of HMGB1 in the chorioamnion after exposure to a proinflammatory stimulus in the chorioamnion makes it an attractive biomarker of intrauterine inflammation. Indeed, Romero et al found increased amniotic fluid levels of HMGB1 in women with preterm labor and/or PROM with intrauterine inflammation compared to those without intrauterine inflammation.19,28 Furthermore, women without intrauterine inflammation but with PROM had a higher HMGB1 levels compared with women in preterm labor alone. In a recent study, Romero et al further demonstrated that women with sterile amniotic inflammation with higher HMGB1 levels have a decreased amniocentesis to delivery interval compared to women with lower amniotic fluid HMGB1 levels.27 Taken together, signaling by HMGB1 in conjunction with proinflammatory cytokines are likely important mediators of chorioamnion injury responses.
A major manifestation of the chorioamnion injury is weakening of the structural integrity of the fetal membranes that may predispose to PROM. Collagen is the predominant structural protein of the fetal membranes, and elegant studies demonstrated decreased collagen expression, increased inflammation, and expression of metalloproteinases causing rupture of membranes at term delivery.37,47 Increased amniotic fluid levels of matrix metalloproteinases (MMPs) 2, 7, 8, and 9 during IA infection suggest decreased collagen abundance contributing to preterm rupture of membranes.11-13,48-53 Additionally, genomic and epigenetic mechanisms have been demonstrated to increase the risk of preterm rupture of membranes in women with variant alleles for altered collagen metabolism or MMPs.54-57 Furthermore, a recent study also showed an altered balance of the proteoglycans biglycan/decorin in fetal membranes from women with chorioamnionitis predicting decreased collagen tensile strength.58 Compared to the uniform collagen fibril staining in the control preterm sheep, we demonstrated dysmorphic or heterogeneous collagen staining by histochemical staining in the chorion and amnion. This effect was particularly evident 24 hours to 4 days after exposure to IA LPS. Consistent with the collagen staining, mRNA expression of collagen 5A1 tended to decrease at 24 and 48 hours after IA LPS. Collagen 1 mRNA expression, reported to be the most abundant collagen species,37 also had a similar profile as collagen 5. Another novel finding was increased collagen in the adventitia of the arterioles in the chorion. These results demonstrate a time-dependent decrease and dysmorphic expression of collagen in the fetal membranes, which could predispose to preterm rupture of membranes.
An unexpected finding was the prominent expression of HMGB1 in the vascular endothelium of the chorion. This finding prompted us to evaluate the chorion for vascular injury and remodeling that may also contribute to injury responses in the fetal membranes. Vascular endothelial injury responses were inferred by increased mRNAs of ICAM-1 and t-PA and a decreasing trend for NOSIII and VEGFR2 mRNA.36,40-42 Coincident with the vascular injury response, increased smooth muscle actin expression indicated arteriolar smooth muscle hypertrophy and remodeling.36 There was no change in the expression of several proangiogenic genes in the chorioamnion after exposure to IA LPS. Our results are consistent with reports of HMGB1 mediating vascular inflammation and injury in other organ systems.59
In this model, we were not able to show that HMGB1 directly caused the injury responses in the chorioamnion. However, its effects in other systems are consistent with our observations. Recent clinical studies demonstrating the strong association of increased amniotic fluid HMGB1 and preterm labor19,27,28 also suggest a role for HMGB1 in the inflammation leading to preterm delivery. It should be noted that the architecture of fetal membrane in the sheep has some dissimilarities compared to humans. In particular, the epitheliochorial placenta in the sheep lacks decidua parietalis, and the chorion laeve of the sheep is much more vascular compared to the chorion laeve of the hemochorial human placenta. Strengths of our work include a comprehensive survey of different DAMPs and a detailed time course of induction of HMGB1 and injury responses in the fetal membranes. Our study has implications for understanding the pathogenesis of preterm rupture of membranes in the context of IA infection/inflammation and underlines the importance of HMGB1 as a potential mediator of inflammation-induced injury response in the chorioamnion. Our study may also help to unravel the pathogenesis of amniotic inflammation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant NIH HD-57869 (SGK).
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014;32(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319(15):972–978. [DOI] [PubMed] [Google Scholar]
- 4. Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007;166(7):786–794. [DOI] [PubMed] [Google Scholar]
- 5. Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis—a complex pathophysiologic syndrome. Placenta. 2010;31(2):113–120. [DOI] [PubMed] [Google Scholar]
- 6. Mi Lee S, Romero R, Lee KA, et al. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. J Matern Fetal Neonatal Med. 2011;24(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonat Med. 2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–1424. [DOI] [PubMed] [Google Scholar]
- 9. Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–681. [DOI] [PubMed] [Google Scholar]
- 10. Simhan HN, Canavan TP. Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG. 2005;112(suppl 1):32–37. [DOI] [PubMed] [Google Scholar]
- 11. Athayde N, Romero R, Gomez R, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8(5):213–219. [DOI] [PubMed] [Google Scholar]
- 12. Fortunato S, Menon R, Lombardi S. Expression of a progelatinase activator (MT1-MMP) in human fetal membranes. Am J Reprod Immunol. 1998;39(5):316–322. [DOI] [PubMed] [Google Scholar]
- 13. Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197(3):292. e291–295. [DOI] [PubMed] [Google Scholar]
- 14. Santolaya-Forgas J, Romero R, Espinoza J, et al. Prelabour rupture of membranes In: Reece AA, Hobbins JC, eds. Clinical Obstetrics: The Fetus and Mothers. Malden, MA: Blackwell; 2006. [Google Scholar]
- 15. Parry S, Strauss JF., III Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–670. [DOI] [PubMed] [Google Scholar]
- 16. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11(1):135–176. [DOI] [PubMed] [Google Scholar]
- 17. Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159(3):661–666. [DOI] [PubMed] [Google Scholar]
- 18. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24(12):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. [DOI] [PubMed] [Google Scholar]
- 23. Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonat Med. 2008;21(7):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. [DOI] [PubMed] [Google Scholar]
- 25. Lu B, Wang H, Andersson U, Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein Cell. 2013;4(3):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012;33(12):633–640. [DOI] [PubMed] [Google Scholar]
- 27. Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol. 2013;37(2):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kallapur SG, Nitsos I, Moss TJ, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179(10):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolfe KB, Snyder CC, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reprod Sci. 2013;20(12):1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kramer BW, Moss TJ, Willet KE, et al. Dose and time response for inflammation and lung maturation after intra-amniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164(6):982–988. [DOI] [PubMed] [Google Scholar]
- 33. Kuypers E, Wolfs TG, Collins JJ, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci. 2013;20(8):946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt AF, Kannan PS, Kemp MW, et al. Intra-amniotic LPS modulates expression of antimicrobial peptides in the fetal sheep lung. Pediatr Res. 2014;76(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuypers E, Willems M, Jellema RK, et al. Responses of the spleen to intra-amniotic lipopolysaccharide (LPS) exposure in fetal sheep. Pediatr Res. 2015;77(1-1):29–35. [DOI] [PubMed] [Google Scholar]
- 36. Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1178–L1185. [DOI] [PubMed] [Google Scholar]
- 37. Lei H, Furth EE, Kalluri R, et al. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest. 1996;98(9):1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meirowitz NB, Smulian JC, Hahn RA, et al. Collagen messenger RNA expression in the human amniochorion in premature rupture of membranes. Am J Obstet Gynecol. 2002;187(6):1679–1685. [DOI] [PubMed] [Google Scholar]
- 39. Berry CA, Nitsos I, Hillman NH, et al. Interleukin 1 in lipopolysaccharide induced chorioamnionitis in the fetal sheep. Reprod Sci. 2011;18(11):1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lindsberg PJ, Carpen O, Paetau A, Karjalainen-Lindsberg ML, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94(5):939–945. [DOI] [PubMed] [Google Scholar]
- 41. Levin EG, Santell L, Osborn KG. The expression of endothelial tissue plasminogen activator in vivo: a function defined by vessel size and anatomic location. J Cell Sci. 1997;110(pt 2):139–148. [DOI] [PubMed] [Google Scholar]
- 42. D’Alquen D, Kramer BW, Seidenspinner S, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57(2):263–269. [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. [DOI] [PubMed] [Google Scholar]
- 44. Dormoy-Raclet V, Cammas A, Celona B, et al. HuR and miR-1192 regulate myogenesis by modulating the translation of HMGB1 mRNA. Nat Commun. 2013;4:2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snyder CC, Wolfe KB, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol. 2013;208(5):399 e391–e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191(4):1346–1355. [DOI] [PubMed] [Google Scholar]
- 47. Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205(3):235. e215–e224. [DOI] [PubMed] [Google Scholar]
- 48. Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstetr Gynecol. 2000;183(1):94–99. [DOI] [PubMed] [Google Scholar]
- 49. Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182(6):1545–1553. [DOI] [PubMed] [Google Scholar]
- 50. Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29(4):308–316. [DOI] [PubMed] [Google Scholar]
- 51. Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12(4):237–246. [DOI] [PubMed] [Google Scholar]
- 52. Strauss JF., III Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci. 2013;20(2):140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Soydinc HE, Sak ME, Evliyaoglu O, et al. Prolidase, matrix metalloproteinases 1 and 13 activity, oxidative-antioxidative status as a marker of preterm premature rupture of membranes and chorioamnionitis in maternal vaginal washing fluids. Int J Med Sci. 2013;10(10):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Romero R, Friel LA, Velez Edwards DR, et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am J Obstet Gynecol. 2010;203(4):361.e361–e361.e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fujimoto T, Parry S, Urbanek M, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277(8):6296–6302. [DOI] [PubMed] [Google Scholar]
- 56. Wang H, Ogawa M, Wood JR, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17(8):1087–1096. [DOI] [PubMed] [Google Scholar]
- 57. Ferrand PE, Parry S, Sammel M, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8(5):494–501. [DOI] [PubMed] [Google Scholar]
- 58. Meinert M, Malmstrom A, Petersen AC, Eriksen GV, Uldbjerg N. Chorioamniontis in preterm delivery is associated with degradation of decorin and biglycan and depletion of hyaluronan in fetal membranes. Placenta. 2014;35(8):546–551. [DOI] [PubMed] [Google Scholar]
- 59. de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11(12):909–917. [DOI] [PubMed] [Google Scholar]