Abstract
Background
Studies on insulin resistance (IR) in chronic kidney disease (CKD) patients are rare, and its exact mechanism remains unclear. In this study, we explored the molecular mechanism of IR with chronic renal failure (CRF) and interventions to alleviate IR in patients with CRF.
Material/Methods
In vivo and in vitro models of CRF were established by 5/6 nephrectomy and urea stimulation C2C12 cells, respectively. Based on the CRF model, angiotensin II (Ang II) and valsartan groups were established to observe the effect of drug intervention on IR. Western blot assays were performed to detect the expression and phosphorylation of IRS-1 and Akt, which are 2 critical proteins in the insulin signaling pathway.
Results
Both urea stimulation and 5/6 nephrectomy induced glucose uptake disorder in skeletal muscle cells (P<0.01). Skeletal muscle IR was aggravated in the Ang II group (P<0.05) but alleviated in the valsartan group (P<0.01). Regardless of the experimental method (in vivo or in vitro), tyrosine phosphorylation of IRS-1 and Akt were significantly lower (P<0.01) and serine phosphorylation was significantly higher (P<0.01) in the model group than in the sham/control group. Compared to the model group, additional Ang II aggravated abnormal phosphorylation (P<0.05); conversely, additional valsartan alleviated abnormal phosphorylation to some extent (P<0.05).
Conclusions
There is skeletal muscle insulin resistance in the presence of CRF. This phenomenon can be aggravated by Ang II and partially relieved by valsartan. One of the mechanisms of IR in CRF patients may be associated with the critical proteins in the IRS-PI3k-Akt pathway by changing their phosphorylation levels.
MeSH Keywords: Insulin Resistance; Kidney Failure, Chronic; Muscle, Skeletal
Background
The prophylaxis and treatment of chronic kidney disease (CKD) has become an important public health issue worldwide in recent years [1]. Without effective control of renal function, CKD patients will ultimately progress into end-stage renal disease (ESRD). Cardiovascular events are the top causes of death in ESRD patients. The incidence of cardiovascular events in dialysis patients has been shown to be 10-fold higher than in the general population [2]. This phenomenon cannot be explained by hypertension, hyperlipidemia, and other traditional risk factors. In the 1980s, DeFronzo et al. [3] observed that insulin resistance (IR) was present in maintenance hemodialysis patients. Our previous study suggested that IR was present in 23% of patients with IgA nephropathy and was associated with the progression of this disease [4]. IR is closely associated with cardiovascular events and is regarded as an independent risk factor for cardiovascular events in ESRD patients [5]. It has been shown that IR in CKD patients is different from that in diabetes patients. The core site of IR in CKD patients is peripheral skeletal muscle [6,7], rather than the liver and adipose tissue. Insulin signaling pathways mainly include the IRS-PI3K-AKT pathway and the IRS-Ras-MAPK pathway. Abnormality in the insulin signaling pathway is thought to directly result in insulin resistance. In the presence of insulin resistance, insulin action through the PI3K pathway is blunted but the MAPK pathway remain intact. Some results indicate that angiotensin II (Ang II) can directly and negatively regulate Akt-dependent insulin signaling and glucose transport activity and impairs insulin signaling in skeletal muscle [8,9]. Angiotensin II receptor blocker can protect mitochondrial function in obese rats with insulin resistance [10] and ameliorates insulin resistance induced by chronic Ang II treatment in rat skeletal muscle [11]. However, there have been few studies on Ang II receptor blockers ameliorating insulin resistance in CKD animal models, and its exact mechanism remains unclear. In the present study, skeletal muscle cells and a rat model of chronic renal failure (CRF) established by a 5/6 nephrectomy were used to explore whether the molecular mechanism of IR with CRF is related to the IRS-PI3K-AKT pathway and to determine the optimal intervention.
Material and Methods
Cells and animals
The C2C12 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences. Male Wistar rats, aged 6–8 weeks and weighing 180–220 g, were purchased from Vital River (Beijing, China). The rats were reared in an SPF-grade animal center for rodents in the General Hospital of the Chinese People’s Liberation Army (PLA). The rearing conditions consisted of a room temperature of 20–25°C, relative humidity of 40–70%, 12 h of light a day, and free access to food and water.
Drugs and reagents
The drugs and reagents included fetal bovine and horse sera (Gibco); DMEM (HyClone); urea and angiotensin II (Ang II) (Sigma); valsartan (Novartis); a 2-deoxyglucose (2-DG) uptake assay kit (Cosmo); a mini-osmotic pump (Alzet); anti-IRS-1, anti-pIRS-1(Tyr612), anti-Akt, and anti-pAkt (Ser473) (Abcam); and anti-pIRS-1 (Ser307) and anti-pAkt (Tyr308) (Cell Signaling Technology).
Cell assay
C2C12 cells were cultured in DMEM containing 10% fetal bovine serum, 1.5 g/L sodium bicarbonate, and 100 U/mL each of penicillin and streptomycin. The cell culture was incubated at 37°C in a humidified (water-saturated) incubator with 5% CO2. The medium was changed every 48 h. When cells reached 80% or higher confluence, the medium was replaced with DMEM containing 2% horse serum to promote cell differentiation and the formation of mature myotubes. The cell culture was available for experiments after 4 days of differentiation. The differentiated cells were randomly divided into negative control, 2-DG, insulin, urea, Ang II, and valsartan groups. The negative control group was continuously supplied with high-glucose DMEM containing 2% horse serum. With the exception of the negative control group, the remaining 5 groups were treated with 2-DG at a final concentration of 1 μM. Insulin at a final concentration of 1 μM was added to the insulin, urea, Ang II, and valsartan groups. Urea at a final concentration of 20 mM was added to the urea, Ang II, and valsartan groups. The final concentrations of Ang II and valsartan were 25 and 10 μM, respectively. Cells were harvested for the subsequent assays after 48 h of culture and stimulation.
For the 2-DG uptake assay, C2C12 cells were allowed to differentiate by conventional culture until myotube structures were formed. The differentiated cells were cultured in serum-free medium for 6 h and stimulated with 1 μM insulin for 18 min, followed by the addition of 2-DG. The cells were lysed and then assayed using a 2-DG uptake assay kit. Direct measurement of the total 2DG-6-phosphate (2DG6P) was performed by an enzymatic assay to determine the cellular glucose uptake [12].
Animal experiments
Thirty-two healthy adult male Wistar rats were randomly and equally divided into sham, model (5/6 nephrectomy), Ang II (5/6 nephrectomy + Ang II), and valsartan (5/6 nephrectomy + Valsartan) groups. All rats had free access to food and water. After 3 days of adaptive feeding, the animals were used to establish a rat model of CRF by a modified 5/6 nephrectomy using the method of Ormrod and Miller [13]. The 5/6 nephrectomy was completed in 2 surgeries. For the sham group, only the adipose capsule was isolated, and kidney tissue was retained. The Ang II group was slowly and continuously treated with 200 ng/kg/min Ang II through a subcutaneously implanted mini-osmotic pump starting on the day of the right nephrectomy [14]. The valsartan group was treated with 20 mg/kg/day valsartan intragastrically, starting on the day of the right nephrectomy [15]. The sham and model groups were intragastrically treated with an equal volume of normal saline. All of the animal experiments were performed in accordance with protocols prescribed by the Animal Experimental Ethics Committee of the Chinese PLA General Hospital and relevant recommendations.
An insulin-tolerance test (ITT) and renal function in rats were assayed prior to and at 4, 8, and 12 weeks after model establishment. Quadriceps tissue samples were collected for Western blot assay.
ITT: The food was withdrawn at the beginning of the experiment and the rats were allowed to drink freely during the experiment. The regular insulin was formulated as 0.4 IU/ml, and each rat was injected intraperitoneally with formulated insulin (0.75 IU/kg) according to body weight. Peripheral blood was collected from the tail of the rats before injection and at 15, 30, 60, and 120 min after injection to test blood glucose. The blood sugar-time curve was plotted according to the measurement results and the insulin sensitivity (IS) was evaluated by the area under the curve (AUC).
Western blot assays of the expression of IRS-1 and Akt phosphorylation
Cells in the different groups were treated for 48 h followed by addition of insulin to a final concentration of 1 μM. The cells were harvested after 20 min of insulin treatment. The skeletal muscle tissues were collected on ice and homogenized. Then, conventional procedures were followed for protein extraction, denaturation, electrophoresis, membrane transfer, hybridization, and color reaction.
Statistical analysis
The statistical analysis was performed using SPSS 19.0. Multiple measures of the same index at different time points were analyzed using multivariate repeated-measures analysis of variance (ANOVA), and comparisons between groups at the same time point were performed using ANOVA for a completely randomized design. Pairwise comparisons of the means between multiple samples were conducted using Dunnett’s t test and the Student-Newman-Keuls (SNK)-q test. Two independent samples with normal distribution and homogeneity of variance were analyzed using a t test. Multiple independent random samples were analyzed using single-factor ANOVA. Two-tailed P values less than 0.05 were considered statistically significant.
Results
2-DG uptake in C2C12 cells with different intervention
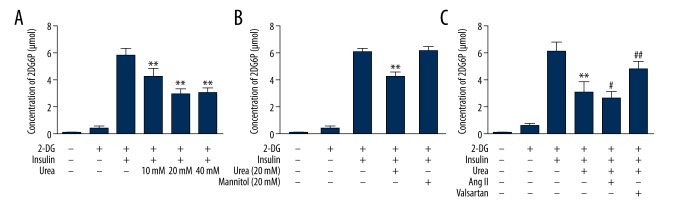
The cells were divided into different groups, and the absorbance of 2DG6P in C2C12 cells was measured 48 h later. Urea concentrations of 10 mM, 20 mM, and 40 mM can decrease 2-DG uptake of C2C12 cells, and the 20 mM urea group decreased the most (P<0.01) (Figure 1A). The 20 mM concentration of urea induced appropriate concentration of C2C12 cell IR, but 20 mM mannitol, used as an osmotic control, had no effect on the concentration of 2DG6P (Figure 1B). Then, each group of differentiated cells was administered the corresponding drugs, and the absorbance of 2DG6P in C2C12 cells in each well was measured after 48 h of incubation. The results showed that 2-DG were significantly increased after insulin stimulation. Urea led to decreased 2-DG uptake in C2C12 cells (P=0.000). Compared to the urea group, the Ang II group obtained a lower concentration of 2DG6P (P=0.040), while the valsartan group had a higher concentration of 2DG6P (P=0.000) (Figure 1C).
Figure 1.
Concentration of 2DG6P in C2C12 cells with different interventions. (A) Dose-response effect of urea on differentiated C2C12 cells. (B) Effect of urea on insulin-stimulated 2-DG uptake in differentiated C2C12 cells. (C) Effect of different interventions on 2-DG uptake in C2C12 cells. Note: compared with the insulin group, ** P<0.01; compared with the urea group, # P<0.05, ## P<0.01.
The general characteristics of each group rats
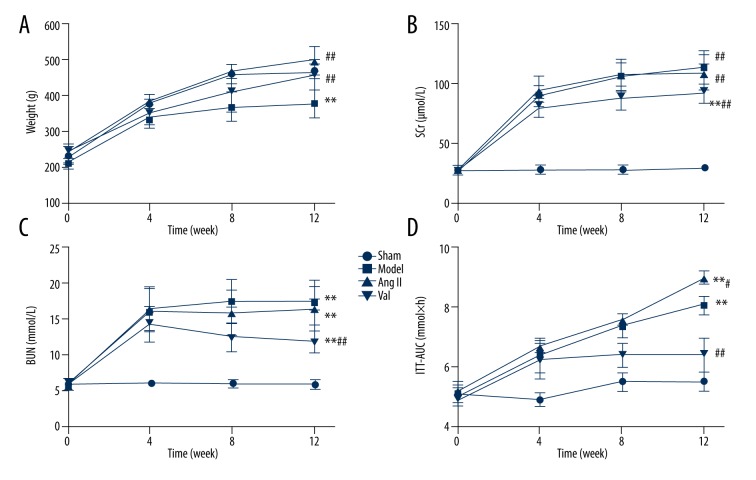
In the multivariate repeated-measures ANOVA, the results showed that the changes of body weight in rats were time-dependent and the effect of time factors was different between groups. The results of the variation between groups was shown. Compared with the sham group, the body weight of the model group, Ang II group, and valsartan group were significantly lower than those of the sham operation group (P <0.05). There was no significant difference between the model group, Ang II group, and valsartan group (P>0.05) (Figure 2A).
Figure 2.
The general characteristics of each group rats. (A) The body weights of the rats were examined every 4 weeks during the 12 weeks of feeding. (B) SCr levels were measured prior to and at 4, 8, and 12 weeks after model establishment. (C) BUN levels were measured prior to and at 4, 8, and 12 weeks after model establishment. (D) ITT in rats was assayed prior to and at 4, 8, and 12 weeks after model establishment. Data are expressed as mean ±SD or as percentages for normal distribution. Compared with the control group, ** P<0.01, compared with the model group, # P<0.05, ## P<0.01 (n=8 rats/group).
The supernatants of abdominal aortic blood were used for serum creatinine (SCr) and blood urea nitrogen (BUN) assays. Significantly higher SCr and BUN levels were detected in the model group than in the sham group (P=0.000). No significant differences were observed between the Ang II and model groups (P>0.05). The SCr and BUN levels in the valsartan group were markedly lower than those in the model group (Figure 2B, 2C).
The results of intragroup variance showed that the area under the curve for the rat ITT (ITT-AUC) tended to change over time, while the effect of the time factor varied in different groups. The results of intergroup variance revealed differences in the ITT-AUC of rats. The model, Ang II, and valsartan groups showed significantly higher ITT-AUC values than in the sham group (P<0.01). Compared to the model group results, the Ang II group obtained a greater ITT-AUC (P=0.047), while the valsartan group had a smaller ITT-AUC (P=0.000) (Figure 2D).
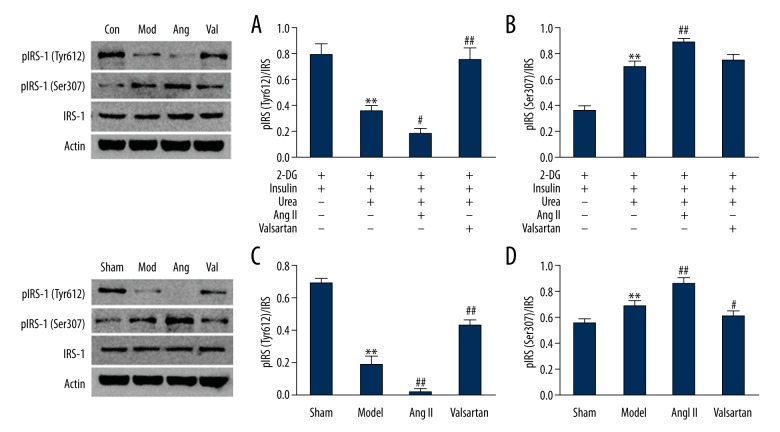
IRS-1, pIRS-1(Tyr612), and pIRS-1(Ser307) expression
The IRS-1 expression levels showed no significant differences between various groups of C2C13 cells or rat skeletal muscle (P>0.05). The CRF/urea stimulation model group had significantly lower pIRS-1(Tyr612)/IRS-1 levels than the sham/control group (P<0.01). Compared to the model group, pIRS-1(Tyr612)/IRS-1 levels were further decreased in the Ang II group (P<0.05) but increased in the valsartan group (P<0.01). The trend of pIRS-1(Ser307)/IRS-1 levels was largely the opposite of the pIRS-1(Tyr612)/IRS-1 levels. The CRF/urea stimulation model group showed significantly higher pIRS-1(Ser307)/IRS-1 levels than in the sham/control group (P<0.01). Compared to the model group, pIRS-1(Ser307)/IRS-1 levels were further increased in the Ang II group (P=0.001); the protein levels of the valsartan group remained unchanged in C2C12 cells (P=0.145) but were significantly decreased in rat skeletal muscle (P<0.05) (Figure 3).
Figure 3.
Immunoblot analysis of insulin-induced phosphorylation of IRS-1. (A) and (B) C2C12 cells; (C) and (D) Skeletal muscle of rats (n=8 rats/group). Compared with the control/sham group, ** P<0.01, compared with the model group, # P<0.05, ## P<0.01.
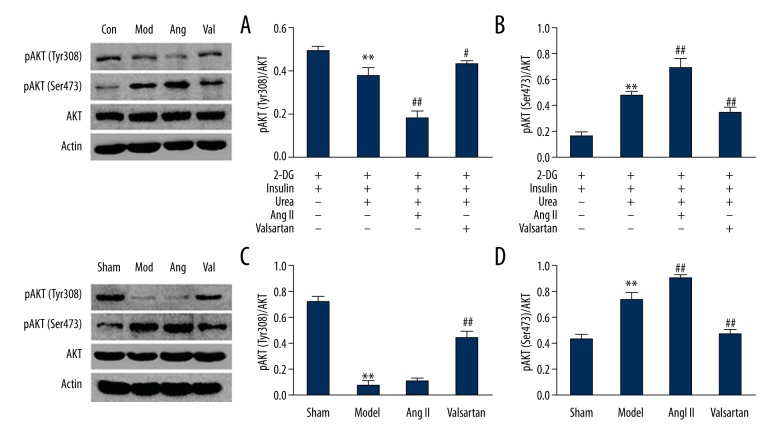
AKT, pAKT (Tyr30 8), and pAKT (Ser473) expression
The AKT expression levels showed no significant differences between various groups of C2C13 cells or rat skeletal muscle (P>0.05). The CRF/urea stimulation model group showed significantly lower pAKT(Tyr308)/AKT levels than in the sham/control group (P<0.01). Compared to the model group, the pAKT(Tyr308)/AKT levels were further increased in the valsartan group (P<0.05); the protein levels of the Ang II group were decreased in C2C12 cells (P=0.000) but exhibited no decreasing trend in rat skeletal muscle (P>0.05). AKT(Ser473)/AKT tended to follow the opposite trend of pAKT(Tyr308)/AKT. The CRF/urea stimulation model group showed significantly higher AKT(Ser473)/AKT levels than in the sham/control group (P<0.01). Compared to the model group, AKT(Ser473)/AKT levels were further increased in the Ang II group (P<0.01) but significantly decreased in the valsartan group, compared to the CRF/urea stimulation model group (P<0.01) (Figure 4).
Figure 4.
Immunoblot analysis of insulin-induced phosphorylation of Akt. (A) and (B) C2C12 cells. (C) and (D) Skeletal muscle of rats (n=8 rats/group). Compared with the sham group, ** P<0.01, compared with the model group, ## P<0.01.
Discussion
Metabolic syndrome has recently received increased research attention due to its close association with increased risk of diabetes [16], cardiovascular disease [17,18], and all-cause mortality [19] in CRF patients. IR, as the core of metabolic syndrome, is considered an independent risk factor for cardiovascular mortality [5,20] in ESRD patients, which is different from other traditional risk factors [21,22]. Canbakan et al. and Thyago Proença de Moraes et al. in their study of prevalent non-diabetic peritoneal dialysis (PD) patients reported that the use of icodextrin in the long-dwell exchanges is associated with reduced insulin resistance as well as fewer metabolic and cardiovascular complications [23,24].
Among various IR-inducing factors, the post-receptor factor is undoubtedly the most important and studied factor. Except for a few special cases [25,26], the vast majority of the numerous known phosphorylation sites on the IRS-1 surface are associated with IR. It has been shown that elevated serum free fatty acid concentrations can lead to decreased IRS-PI3K-Akt pathway activity and induce IR in skeletal muscle [27–29], and the inhibition of normal tyrosine phosphorylation of IRS-1 plays an important role in this process [30].
The results of this study indicated that urea stimulation could induce IR in skeletal muscle. In the animal experiments, rats of the model group presented with renal function deterioration and significant ITT-AUC increases compared to the sham group. Regardless of the in vivo or in vitro conditions, the model group showed significantly lower tyrosine phosphorylation levels but higher levels of serine phosphorylation of IRS-1 and Akt compared to the sham/insulin control group. These changes led to a decline in the overall IRS-PI3K-Akt pathway activity, which first revealed the primary mechanism of IR in CRF.
Due to a partially shared pathway between Ang II and insulin signaling pathways, Ang II stimulation can increase Ser phosphorylation [31,32] but decrease Tyr phosphorylation of IRS-1 [33], thereby reducing insulin-mediated IRS-PI3k-Akt pathway activity. We found that plasma Ang II levels were increased in the model group (sham group 60.1±7.5 ng/L vs. model group 150.5±14.3 ng/L, P<0.05) in the preliminary experiment. The present study suggests that after urea stimulation, additional Ang II led to further reduction of 2-DG uptake in muscle cells. In animal experiments, administration of Ang II by an osmotic pump resulted in significantly lower IS in CRF rats. According to the results of a Western blot assay, Ang II led to increased abnormal phosphorylation in skeletal muscle. On the other hand, angiotensin receptor blocker (ARB) drugs, such as valsartan, act on the Ang II receptor 1 (AT1) and stimulate the overall enhancement of IRS-1 and its downstream insulin-signaling pathway activity through a common pathway [34]. The present study experimentally demonstrated such an effect of valsartan; valsartan partially diminished the reduction of 2-DG uptake in muscle cells and decreased the ITT-AUC in rats as induced by urea stimulation. To some extent, valsartan also mitigated abnormal phosphorylation in the tissue. This finding is consistent with our conclusion from a previous meta-analysis [35].
Insulin-sensitizing agents commonly used in clinics primarily consist of thiazolidinediones (TZDs) [36]. TZDs are effective as insulin sensitizers, and have been reported to reduce all-cause mortality in non-insulin-dependent diabetes mellitus and hemodialysis patients [37]. However, TZDs also have serious problems; due to their obvious water-sodium retention effect, it has been noted that TZDs may increase the risk of heart failure [38] and cardiovascular mortality in patients [39]. Moreover, TZDs may increase the risk of bladder cancer [40] and thus are unsuitable for CKD patients. In contrast, ARBs can protect renal function and reverse cardiac remodeling, which is undoubtedly a safe and optimal choice for alleviating IR in ESRD patients.
Conclusions
The present study established in vivo and in vitro models of urea-induced IR. The in vivo experiments demonstrated that valsartan could alleviate IR in a CRF model. Furthermore, the mechanism of urea-induced IR was explored. Urea stimulation induced IR in skeletal muscle cells, and CRF rats exhibited IR. The mechanism of this phenomenon may be related to abnormal phosphorylation and activity inhibition of the IRS-PI3K-Akt pathway. Because Ang II and insulin share some common signaling pathway, valsartan as an ARB drug can alter the abnormal phosphorylation of important molecules in the IRS-PI3k-Akt pathway and thereby alleviate IR.
Footnotes
Source of support: This work was supported by grants from the National Sciences Foundation of China (grant numbers 81471027, 81273968, 81072914, and 81401160), the Ministerial Projects of the National Working Commission on Aging (grant number QLB2014W002), The Four hundred project of 301 (grant number YS201408), and the Beijing Nova Program (grant number Z161100004916129)
References
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–19. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Smith D, Alvestrand A. Insulin action in uremia. Kidney Int Suppl. 1983;16:S102–14. [PubMed] [Google Scholar]
- 4.Yang Y, Wei RB, Wang YD, et al. Higher HOMA-IR index and correlated factors of insulin resistance in patients with IgA nephropathy. Clin Nephrol. 2012;78(5):353–58. doi: 10.5414/CN107613. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(7):1894–900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- 6.Pham H, Utzschneider KM, de Boer IH. Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertensi. 2011;20(6):640–46. doi: 10.1097/MNH.0b013e32834b23c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao MT, Sung CC, Hung KC, et al. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369. doi: 10.1155/2012/691369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond-Stanic MK, Henriksen EJ. Direct inhibition by angiotensin II of insulin-dependent glucose transport activity in mammalian skeletal muscle involves a ROS-dependent mechanism. Arch Physiol Biochem. 2010;116(2):88–95. doi: 10.3109/13813451003758703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Sowers JR, Nistala R, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281(46):35137–46. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 10.Thorwald M, Rodriguez R, Lee A, et al. Angiotensin receptor blockade improves cardiac mitochondrial activity in response to an acute glucose load in obese insulin resistant rats. Redox Biol. 2018;14:371–78. doi: 10.1016/j.redox.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lastra G, Santos FR, Hooshmand P, et al. The novel angiotensin II receptor blocker azilsartan medoxomil ameliorates insulin resistance induced by chronic angiotensin II treatment in rat skeletal muscle. Cardiorenal Med. 2013;3(2):154–64. doi: 10.1159/000353155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito K, Lee S, Shiuchi T, et al. An enzymatic photometric assay for 2-deoxyglucose uptake in insulin-responsive tissues and 3T3-L1 adipocytes. Anal Biochem. 2011;412(1):9–17. doi: 10.1016/j.ab.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Ormrod D, Miller T. Experimental uremia. Description of a model producing varying degrees of stable uremia. Nephron. 1980;26(26):249–54. doi: 10.1159/000181994. [DOI] [PubMed] [Google Scholar]
- 14.Ogihara T, Asano T, Ando K, et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2003;40(6):872–79. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 15.Shiuchi T, Iwai M, Li HS, et al. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension. 2004;43(5):1003–10. doi: 10.1161/01.HYP.0000125142.41703.64. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care. 2003;26(11):3153–59. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 17.Ninomiya JK, L’Italien G, Criqui MH, et al. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109(1):42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 18.Kachur S, Morera R, De Schutter A, Lavie CJ. Cardiovascular risk in patients with prehypertension and the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):15. doi: 10.1007/s11906-018-0801-2. [DOI] [PubMed] [Google Scholar]
- 19.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(7):1894–900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- 21.Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: A hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8(3):475–86. doi: 10.1681/ASN.V83475. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58(1):353–62. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 23.Canbakan M, Sahin GM. Icodextrine and insulin resistance in continuous ambulatory peritoneal dialysis patients. Renal Fail. 2007;29(3):289–93. doi: 10.1080/08860220601166271. [DOI] [PubMed] [Google Scholar]
- 24.de Moraes TP, Andreoli MC, Canziani ME, et al. Icodextrin reduces insulin resistance in non-diabetic patients undergoing automated peritoneal dialysis: Results of a randomized controlled trial (STARCH) Nephrol Dial Transplant. 2015;30(11):1905–11. doi: 10.1093/ndt/gfv247. [DOI] [PubMed] [Google Scholar]
- 25.Ormrod F. Chronic lead poisoning occurring in the manufacture of steel. Br Med J. 1983;1(1694):1264. doi: 10.1136/bmj.1.1694.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ormrod AN. Fatal ingestion of tintacks by chimpanzee. Vet Rec. 1950;62(28):408. doi: 10.1136/vr.62.28.408. [DOI] [PubMed] [Google Scholar]
- 27.Irving JG, Ormrod JK. The value of a health service to the insurance company. Proc Annu Meet Med Sect Am Life Conv. 1959;47:44–57. [PubMed] [Google Scholar]
- 28.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–74. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 29.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1 – associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–59. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ormrod JN. Diverticulum of the lacrimal sac. Br J Ophthalmol. 1958;42(42):526–28. doi: 10.1136/bjo.42.9.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniyama Y, Hitomi H, Shah A, et al. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25(6):1142–47. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 32.Andreozzi F, Laratta E, Sciacqua A, et al. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94(9):1211–18. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 33.Folli F, Kahn CR, Hansen H, et al. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100(9):2158–69. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horiuchi M, Mogi M, Iwai M. Signaling crosstalk angiotensin II receptor subtypes and insulin. Endocr J. 2006;53(1):1–5. doi: 10.1507/endocrj.53.1. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Wei RB, Xing Y, et al. A meta-analysis of the effect of angiotensin receptor blockers and calcium channel blockers on blood pressure, glycemia and the HOMA-IR index in non-diabetic patients. Metabolism. 2013;62(12):1858–66. doi: 10.1016/j.metabol.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53(Suppl 1):S60–65. doi: 10.2337/diabetes.53.2007.s60. [DOI] [PubMed] [Google Scholar]
- 37.Ormrod R. Legality of research on children. Lancet. 1978;2(8095):888. doi: 10.1016/s0140-6736(78)91586-6. [DOI] [PubMed] [Google Scholar]
- 38.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: Systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. doi: 10.1136/bmj.d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ormrod DJ, Miller TE. Complement-mediated immune mechanisms in renal infection. Clin Exp Immunol. 1978;33(1):115–21. [PMC free article] [PubMed] [Google Scholar]
- 40.Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: Nested case-control study. BMJ. 2012;344(5):e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]