Abstract
Caffeoylserotonin (CaS), one derivative of serotonin (5-HT), is a secondary metabolite produced in pepper fruits with strong antioxidant activities. In this study, we investigated the effect of CaS on proliferation and migration of human keratinocyte HaCaT cells compared to that of 5-HT. CaS enhanced keratinocyte proliferation even under serum deficient condition. This effect of CaS was mediated by serotonin 2B receptor (5-HT2BR) related to the cell proliferation effect of 5-HT. We also confirmed that both CaS and 5-HT induced G1 progression via 5-HT2BR/ERK pathway in HaCaT cells. However, Akt pathway was additionally involved in upregulated expression levels of cyclin D1 and cyclin E induced by CaS by activating 5-HT2BR. Moreover, CaS and 5-HT induced cell migration in HaCaT cells via 5-HT2BR. However, 5-HT regulated cell migration only through ERK/AP-1/MMP9 pathway while additional Akt/NF-κB/MMP9 pathway was involved in the cell migration effect of CaS. These results suggest that CaS can enhance keratinocyte proliferation and migration. It might have potential as a reagent beneficial for wound closing and cell regeneration.
Keywords: Caffeoylserotonin, Cell migration, G1 progression, Serotonin 2B receptor, Serotonin
INTRODUCTION
Caffeoylserotonin (CaS), one of hydroxycinnamic acid amide derivatives of serotonin (5-HT), has been detected in pepper fruits as a secondary metabolite (1). CaS and 5-HT both possess strong radical scavenging activities. They can reduce intracellular ROS generation, lipid peroxidation, and oxidative stress-induced cell death in HepG2 and HaCaT cells (2). CaS protects against oxidative stress-induced cell death through activating Nrf2-mediated HO-1 induction via PI3K/Akt and/or PKC pathways in HaCaT cells (3).
Skin is the first line of defense of our immune system. Innate immune cells, neutrophils, and macrophages will immediately secrete reactive oxygen species (ROS) after wounding to protect the tissue against invading pathogens, chemicals, injury, and UV (4). However, ROS may contribute to chronic and non-healing wounds. Low levels of ROS can inhibit the migration and proliferation of keratinocytes (5) whereas excessive amounts of ROS can lead to severe cell damage, premature aging, and cancer (6). Currently, there are strong evidences supporting the role of oxidative stress in the pathogenesis of chronic and non-healing ulcers (7). In this respect, several antioxidant reagents such as ascorbic acid, tocopherols, allopurinol, and other natural compounds have shown positive effects in improving wound repair process or preventing aging of damaged tissues (8–10). However, it is currently unclear whether CaS might have potential as a reagent to improve cell proliferation and wound healing process in damaged human skin tissue.
Therefore, the objective of this study was to investigate the effect of CaS on proliferation and migration of human keratinocyte HaCaT cells compared to that of 5-HT. Interestingly, CaS promoted cell proliferation and cell migration even under serum deficient condition. We confirmed that such effect of CaS was mediated by serotonin 2B receptor (5-HT2BR) which was also associated with cell proliferation effect of 5-HT. Several reports have demonstrated that 5-HT can act as a mitogen mediated by 5-HT2BR/ERK pathway (11, 12). We also confirmed that CaS and 5-HT both could induce G1 progression and cell migration via 5-HT2BR/ERK pathway in HaCaT cells. In addition, we found that CaS had an additional Akt pathway to upregulate expression levels of cyclin D1, cyclin E and MMP9 by activating 5-HT2BR.
RESULTS
Effect of CaS on cell cycle progression and cell cycle regulators in HaCaT cells
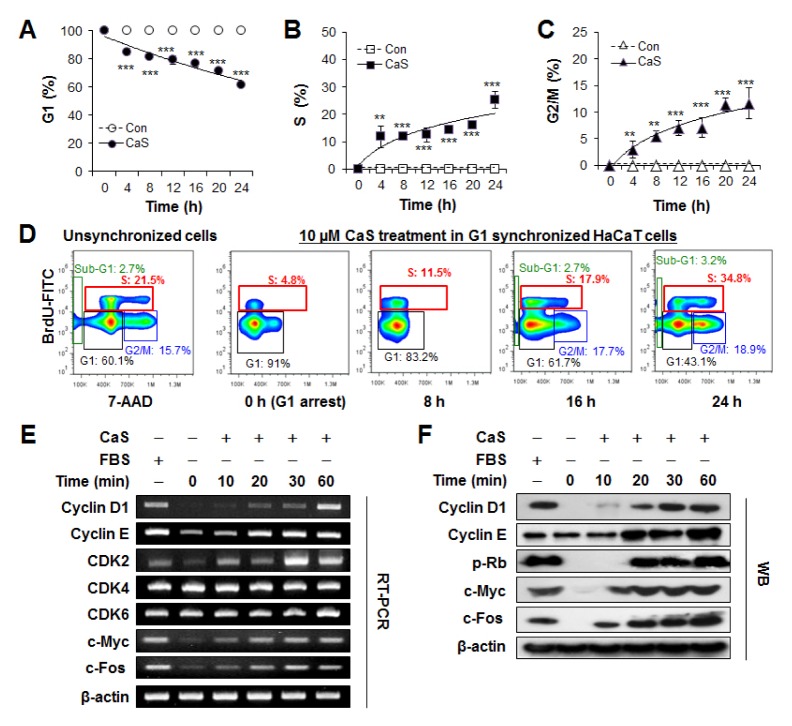
To investigate whether CaS could enhance keratinocyte proliferation, we first examined its impact on cell cycle kinetics in human keratinocyte HaCaT cells. Unsynchronized HaCaT cells showed canonic distribution in G1, S, and G2/M phases. However, after 48 h of serum deprivation, cell cycle progression was significantly suppressed and most cells were synchronized at G1/S check point (S3). After adding 10 μM CaS into G1 synchronized cells, the percentage of HaCaT cells in G1 phase was decreased (from 100% to 61.8 ± 1.3%, P < 0.005, Fig. 1A). They were accumulated at S phase (from 0 to 25.3 ± 3.2%, P < 0.005, Fig. 1B) and G2/M phase (from 0 to 11.7 ± 2.8%, P < 0.005, Fig. 1C) compared to untreated control which was unchanged. These results demonstrated that CaS clearly attributed to cell cycle progression in HaCaT cells. Cell cycle analysis only determines the proportion of cell cycle phase without giving an index of cell proliferation. As a complementary approach to examine cell proliferation, anti-BrdU-FITC/7-AAD staining was performed to measure the effect of 10 μM CaS on DNA replication (Fig. 1D). In CaS-stimulated G1-arrested HaCaT cells, cell proportions of S and G2/M phases were gradually increased even under serum-deficient condition. Therefore, we concluded that CaS could promote cell proliferation in human keratinocytes in a time-dependent manner.
Fig. 1.
CaS attributes to cell cycle progression in HaCaT cells. (A–C) Kinetic FACS profiles of G1-arrested HaCaT cells for 24 h in the presence (filled symbols) or absence (empty symbols) of CaS under serum deprivation. **P < 0.05, and ***P < 0.005 vs. untreated control. (D) Effect of CaS on cell proliferation was monitored using BrdU and 7-AAD double staining. Percentages of cells in sub-G1 (green), G1 (black), S (red), and G2/M (blue) phase are indicated in each flow cytometric dot plot. (E) RT-PCR analyses for cyclin D1, cyclin E, c-myc, c-fos for indicated time. (F) Western blotting analyses for cyclin D1, cyclin E, p-Rb, c-myc, c-fos for indicated time. β-actin was used as a loading control.
Moreover, we analyzed expression levels of key molecules that could control G1 phase progression of cells by CaS. As shown in Fig. 1E, mRNA levels of cyclin D1, cyclin E, and CDK2 were increased by CaS stimulation in a time-dependent manner. Moreover, mRNA levels of transcriptional factors c-Myc and c-Fos were increased by CaS stimulation in a time-dependent manner, in correlation with cell proliferation. However, mRNA level of CDK4 or and CDK6 was not significantly changed after CaS stimulation. Consistent with RT-PCR results, protein expression levels of cyclin D1, cyclin E, c-Myc, and c-Fos were also increased in a time-dependent manner after CaS stimulation. In addition, p-Rb protein appeared at 20 min after CaS stimulation (Fig. 1F).
CaS triggers G1 progression via 5-HT2BR/ERK and Akt pathway
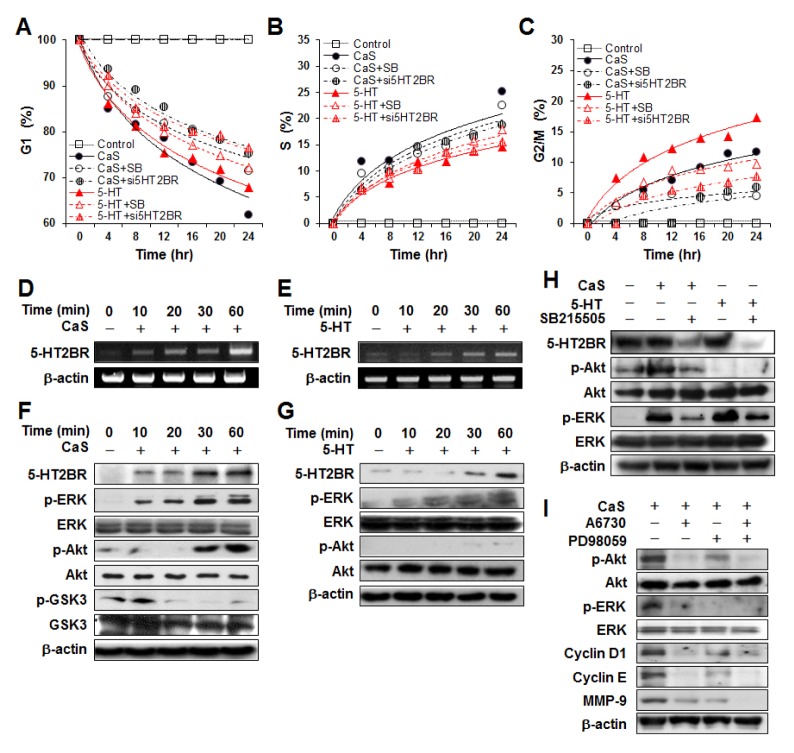
Among 13 serotonin receptors (5-HTRs) subtypes, 5-HT2BR and 5-HT7R variants are generally detected in normal and pathologic human keratinocytes (13). In this study, we also confirmed that 5-HT2BR mRNA was one main gene of serotonin receptors expressed in HaCaT cells under basal culture condition (S2, S4). Next, after treating HaCaT cells with CaS or 5-HT, we determined mRNA expression profiles of 5-HT2BR (Fig. 2D and 2E). Expression levels of 5-HT2BR mRNA were increased in a time-dependent manner by the same dose (10 μM) of CaS or 5-HT. The 5-HT2BR protein levels was also increased in CaS or 5-HT treated cells, in agreement with RT-PCR results (Fig. 2F and 2G, first lines). Under 5-HT treatment condition, phosphorylation of ERK was stimulated in a time-dependent manner while phosphorylation of Akt was observed only at detectable level (Fig. 2G). However, CaS activated p-ERK and p-Akt pathways, with p-ERK being observed at earlier time than p-Akt under CaS treatment (Fig. 2F). We also examined expression levels of p-GSK3 and GSK3 to clarify the effect of CaS on Akt/GSK3/cyclin D1 pathway. A decrease in p-GSK3 was noted in CaS treated cells compared to an increase in p-Akt (Fig. 2F) or cyclin D1 (Fig. 1F). This result suggested that Akt/GSK3 pathway was also involved in the stimulation of cyclin D1 expression by CaS. There was no significant alteration in JNK or p38 pathway after treatment with 5-HT or CaS (S5).
Fig. 2.
Effect of inhibitor (SB) and siRNA for 5-HT2BR on G1 progression induced by CaS or 5-HT. (A–C) SB and siRNA treatment induced delay of G1 release (B) and increase of G2/M population (D) without significant change in S population (C). RT-PCR analysis of 5-HT2B receptor in HaCaT cells after treatment with CaS (D) or 5-HT (E) for indicated time periods. (F) Western blotting analysis of 5-HT2B, p-Akt, Akt, p-ERK, ERK, p-GSK3, and GSK3 after CaS treatment. p-GSK3 level was decreased while p-ERK and p-Akt levels were increased in a time-dependent manner. (G) Western blotting analysis for 5-HT2BR, p-Akt, Akt, p-ERK, and ERK after 5-HT treatment. p-Akt was observed only at detectable level. (H) Cells were treated with SB215505 (10 μM) to inhibit 5-HT2BR for 30 min before treatment with 10 μM of 5-HT or CaS. SB215505 blocked ERK and Akt phosphorylation induced by CaS or 5-HT. (I) Cells were treated with A6730 (50 μM) to inhibit Akt and/or PD98059 (50 μm) to inhibit MEK-1 for 30 min before treatment with 10 μM CaS for 1 h.
Effect of 5-HT2BR inhibition on G1 progression mediated by CaS or 5-HT
To clarify that 5-HT2BR was a mediator of G1 progression activated by 5-HT or CaS, we investigated the effect of SB212205, a selective inhibitor of 5-HT2BR, on 5-HT or CaS-mediated G1 progression and related protein expression. FACS analysis showed that CaS or 5-HT treatment stimulated the progression from G1 to S and G2/M phases and that SB212205 could partially offset their effects on the G1 progression (Fig. 2A–C, S3). As shown in Fig. 2A, CaS treated cells showed the most pronounced reduction effect on G1 population, followed by 5-HT treated cells, SB215505 treated cells before treatment with 5-HT, and SB215505 treated cells before treatment with CaS. G1 reduction might be accompanied by accumulations of sub-S or G2/M population. S population was also observed in the same condition as that for G1 population (Fig. 2B). The most pronounced effect in S population was its increase in CaS treated cells. This was reasonable because it was accompanied by the reduction of G1 population in CaS treated cells. Interestingly, the increase rate of S population in 5-HT treated cells was significantly lower than that in other treated cells. However, the population of G2/M in 5-HT treated cells was more rapidly accumulated compared to that in other treated cells (Fig. 2C). Consistent with results with SB212205, cell cycle releasing was clearly inhibited by 5-HT2BR-specific siRNA transfection (Fig. 2A–C, S3). These results suggested G1 progression strongly mediated through 5-HT2BR in CaS or 5-HT stimulated conditions. Moreover, we used SB212205 to determine whether the activation of ERK and Akt pathways by CaS was via 5-HT2BR. As shown in Fig. 2H, both expression levels of p-ERK and p-Akt were down-regulated in HaCaT cells by pretreatment with 10 μM SB212205 for 30 min before CaS treatment.
To investigate which was the major pathway for cyclin D1 expression induced by CaS, we used inhibitors of Akt and/or MEK-1 to pretreat cells for 30 min before CaS treatment (Fig. 2I). In cells treated with CaS only, expression of p-Akt, p-ERK, cyclin D1, and cyclin E was clearly observed. However, 30 min of pretreatment of cells with A6730 (50 μM), a selective inhibitor of Akt, completely abolished the expression of p-ERK, cyclin D1, and cyclin E evaluated at the same time point. PD98059 (50 μM), a selective inhibitor of MEK-1, reduced expression levels of CaS-induced p-Akt, cyclin D1, and cyclin E without abolishing their levels. These resultes showed that Akt can be major pathway for cyclin D1 expression.
5-HT is more effective than CaS for G2/M progression
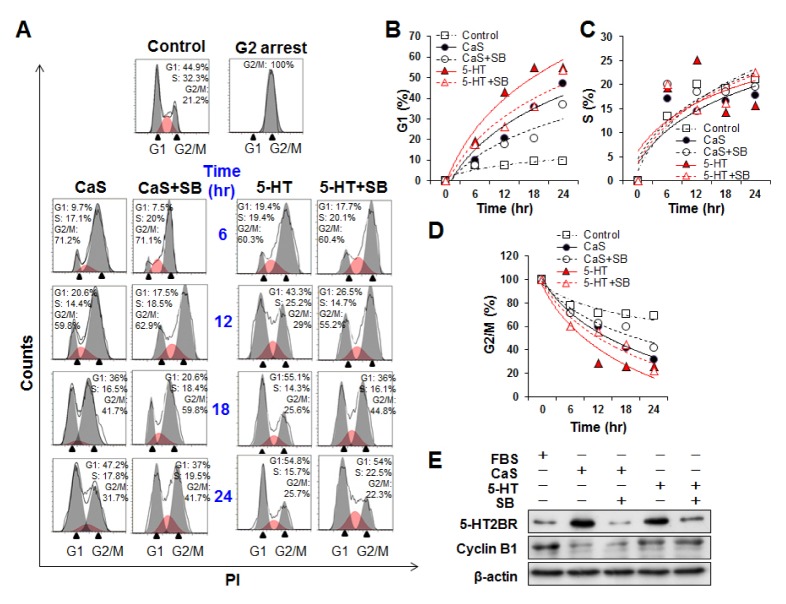
As shown in Fig. 2, CaS induced G1 progression slightly faster than 5-HT whereas 5-HT accelerated the reduction of S portion and the accumulation of G2/M portion more rapidly than CaS. These results led to a hypothesis that effects of CaS and 5-HT were different depending on the stage of cell cycle. 5-HT might promote S and/or G2/M phase more effectively compared to CaS. To address these possibilities, HaCaT cells were treated with nocodazole for 16 h to induce G2 arrest (Fig. 3A). After removing nocodazole, cell cycle was released with CaS or 5-HT. As shown in Fig. 3, part of synchronized cells at G2/M phase progressed to G1 phase and started DNA synthesis after treatment with CaS or 5-HT. Both 5-HT and CaS treated cells initiated and completed S phase with very similar kinetics. However, 5-HT was more effective than CaS in initiating S phase in HaCaT cells (Fig. 3C). We also wondered if G2/M progression was affected by 5-HT2BR. Cells pretreated with SB212205 before CaS or 5-HT treatment showed delays in G2/M progression compared to cells treated with CaS or 5-HT (Fig. 3D). After remove nocodazole, G2/M synchronized cells progressed into cell cycle without CaS or 5-HT, but the levels of G1 progressed cells was less than 10% even after 24 h whereas the levels of G1 progressed cells were reached about 50% under CaS or 5-HT (Fig. 3B and S6). Activation of the Cyclin B1/Cdk1 complex is necessary for the progression of cells from G2 to M phase (14). Therefore, we investigated the effect of CaS and 5-HT in activating cyclin B1. More elevated levels of Cyclin B were observed in 5-HT treated cells compared to those in CaS treated cells (Fig. 3E). This observation suggested that 5-HT accelerated G2/M progression in cells more than CaS. We also investigated whether 5-HT2BR regulates the expression of cyclin B. However, there were no significant differences in cyclin B level between cells pretreated with SB215505 and cells without such pretreatment, indicating that 5-HT2BR was not involved in the regulation of cyclin B.
Fig. 3.
Effects of 5-HT2BR inhibitor (SB) on G2/M progression induced by CaS or 5-HT. (A) HaCaT cells synchronized at G2/M arrest by nocodazole were pretreated with 10 μM SB215505 before treatment with CaS and 5-HT for 24 h. These cells were then fixed and stained with PI and analyzed by FACS. (B–D) SB215505 treatment induced delay of G1 release (B) and increase G2/M population (D) without causing significant change in S population (C). Filled symbols represent CaS or 5-HT treatment only. Empty symbols represent cells pre-treated with SB215505 before 5-HT or CaS treatment. (E) Cells were pre-treated with SB215505 (10 μM) to inhibit 5-HT2BR for 30 min before treatment with 10 μM of 5-HT or CaS. After 6 h of treatment with 5-HT or CaS, cell lysates were collected and cyclin B protein levels were measured by western blotting.
CaS and 5-HT enhance cell migration via 5-HT2BR
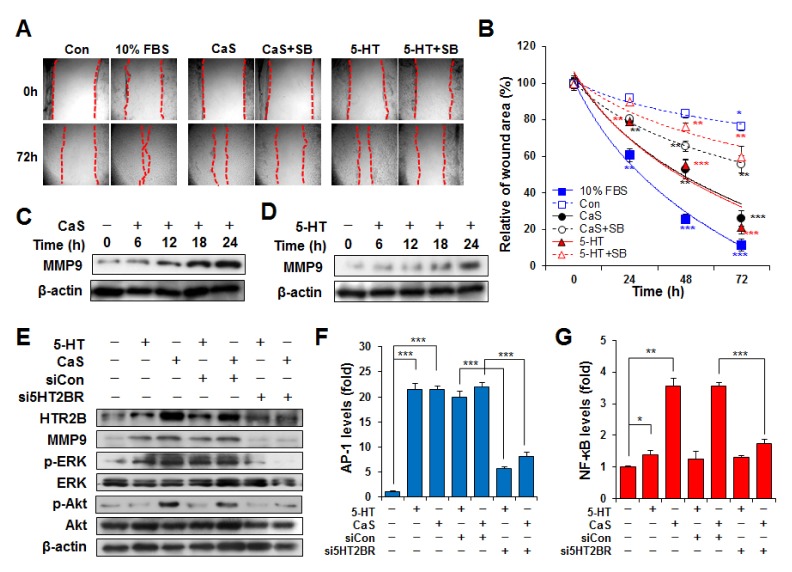
Migration abilities of CaS or 5-HT treated cells were determined in wounded space on culture plates for 72 h (Fig. 4). CaS or 5-HT induced migration of HaCaT cells to a greater extent compared to control treatment. There was no significant difference in cell migration rate between 5-HT and CaS treatment groups. Cell migration rates were significantly decreased by pre-treatment with SB2122055, a HT2BR inhibitor, in both CaS and 5-HT induced dynamics (Fig. 4A and 4B, S7). After 72 h of incubation, significantly (2.1-fold) higher cell migration rate was observed in CaS treated cells without SB215505 pretreatment compared to that of cells pre-treated with SB215505 followed by CaS treatment. Similarly, significantly (2.8-fold) higher cell migration rate was observed in 5-HT treated cells without SB215505 pretreatment compared to that of cells pre-treated with SB215505 followed by 5-HT treatment. These results suggested that migration of 5-HT-induced cells was inhibited more by SB215505 than that of CaS-induced cells.
Fig. 4.
Effect of CaS or 5-HT on cell migration. (A) HaCaT cells were pretreated with 10 μM SB215505 (SB). HaCaT monolayer was then scratched (dotted line) with 1 mL pipette tip and cultured with 5-HT or CaS for indicated time period. Magnification, x20. (B) Level of cell migration into the wound scratch was quantified as percentage of open area. Blue square represents cells incubated with 10% FBS as positive control. Empty blue squares represent untreated control. Circle symbols represent cells treated with CaS. Triangle symbols represent cells treated with 5-HT. Empty circle and triangle symbols represent cell pre-treated with SB215505 before 5-HT and CaS treatment, respectively. Values represent averages ± SE of three independent measurements along the wound scratch. *P < 0.1, **P < 0.01, and ***P < 0.001 vs. untreated control at the same time point. (C–D) CaS and 5-HT induced MMP9 expression level in a time-dependent manner. Un-transfected cells or cells transiently transfected with siCon or si5HT2BR were cultured under CaS or 5-HT. Cells were harvested at 24 h after transfection and assayed. 5-HT2BR siRNA transfection inhibited MMP9 protein expression (E) as well as AP-1 (F) and NF-κB (G) levels.
In addition, both CaS and 5-HT increased MMP-9 expression in a time-dependent manner (Fig. 4C and D). It is well-known that mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase/protein kinase B (PI3K/Akt) pathways mediate MMP-9 release (15). Therefore, we examined whether 5-HT2BR affected expression levels of MMP-9, signaling proteins (Akt and ERK), and transcription factors (AP-1 and NF-κB). Consistent with results shown in Fig. 2H, levels of p-ERK (induced by both 5-HT and CaS) and p-Akt (induced by CaS only) and expression of MMP-9 were clearly inhibited by 5-HT2BR-specific siRNA transfection (Fig. 4E). Since the MMP-9 promoter contains AP-1 and NF-κB binding site, loss of AP-1 or NF-κB activation is expected to result in reduced MMP-9 gene expression and cell migration. AP-1 was activated by both CaS and 5-HT, NF-κB was activated only by CaS. Transfection with siRNA targeting HTR2B also decreased CaS- or 5-HT-induced AP-1 and NF-κB activation (Fig. 4F and 4G). This means that 5-HT activates only p-ERK/AP-1/MMP-9 pathway, whereas CaS can additionally activate p-Akt/NF-κB/MMP-9 pathway for cell migration.
DISCUSSION
It has been reported that 5-HT can lead to cell proliferation via 5-HT2BR in fibroblasts (11), β-cells (16), adult rat hepatocytes (17), and osteoblasts (18). CaS is one derivate of serotonin (5-HT). It could activate 5-HT2BR in HaCaT cells in a dose-dependent manner (Fig. 2D). Serotonin actions are mediated through interactions with membrane-bound receptors that can be categorized into seven families with at least 21 subtypes. Among those subtypes, 5-HT2BR and 5-HT7R variants were detected in both normal and pathologic human keratinocytes in our study whereas 5-HT2BR mRNA was found in the majority of HaCaT cells (S4). Based on these results, we hypothesized that CaS could enhance keratinocyte proliferation by mimicking 5-HT-induced proliferation via 5-HT2BR.
To test our hypothesis, we added CaS into HaCaT cells synchronized in G1 phase by serum starvation and measured changes in G1 population. As shown in Fig. 1, CaS progressed G1-synchronized cells (pass through G1 check point) into S and G2/M phases even under serum and growth factors deficit conditions. In addition, we confirmed that 5-HT activated the ERK pathway downstream of 5-HT2BR. However, CaS not only activated ERK, but also activated Akt. It also inhibited phosphorylation of GSK3 (Fig. 2F). GSK3 is the primary kinase that phosphorylates cyclin D1. Phosphorylated cyclin D1 is then translocated from the nucleus to the cytoplasm where it is degraded through the ubiquitin-dependent proteolysis pathway (19). Akt directly phosphorylates and inactivates GSK3. GSK3-induced cyclin D1 degradation is inhibited by the activation of Akt pathway (20). Moreover, we investigated that both cyclin D1 and cyclin E were more strongly inhibited by Akt inhibitor than those by ERK inhibitor (Fig. 2I). This suggests that G1 progression induced by CaS is regulated by Akt more than that by ERK. In addition, we found that CaS and 5-HT had different effects depending on the stage of cell cycle. CaS induced faster G1 progression than 5-HT in G1 synchronized cells (Fig. 2A). And G2/M population was more rapidly decreased after 5-HT treatment than that after CaS treatment in G2 synchronized cells (Fig. 3D). In addition, 5-HT shortened the initiation time of S phase (Fig. 3C). All cell cycle progressions were postponed by 5-HT2BR inhibitor (Fig. 2 and 3), suggesting that both CaS and 5-HT could induce cell cycle progression through 5-HT2BR in HaCaT cells.
We also compared cell migration rates between CaS and 5-HT treatment conditions. There were no significant differences in cell migration rate between 5-HT and CaS treatments. However, keratinocyte migration after 5-HT treatment was more sensitive to 5-HT2BR inhibition than that after CaS treatment (Fig. 4B). Moreover, 5-HT regulated cell migration only through the ERK/AP-1/MMP9 pathway whereas CaS regulated cell migration through both ERK/AP-1/MMP9 and Akt/NF-κB/MMP9 pathways (Fig. 4F and G). This suggests that 5-HT2BR can more strongly regulate G1 progression induced by CaS than that by 5-HT. Interestingly, both 5-HT and CaS significantly induced the expression level of 5-HT2BR (Fig. 2). Recently, it confirmed that 5-HT stimulated the expression of 5-HT2BR via an AP-1-dependent mechanism in pulmonary artery smooth muscle cells (PASMCs) and hepatic cells (21, 22). The promoter region of the human 5-HT2BR gene has several putative AP-1 motifs and NF-κB motifs (S8). It suggest that 5-HT can stimulate the expression of 5-HT2BR via an AP-1-dependent mechanism and CaS may stimulate 5-HT2BR expression through both only AP-1 also NF-κB (Fig. 4F and 4G).
In summary, we examined the effect of CaS on cell proliferation and migration in HaCaT cells compared to that of 5-HT. Our results demonstrated that cell proliferation and migration were mediated by 5-HT2BR under CaS and 5-HT treatment conditions. CaS further activated Akt pathway to regulate cell proliferation and migration in addition to 5-HT2BR/ERK pathway (S9). Our results provide novel insights into the valuable benefit of CaS and 5-HT for skin wound closure and anti-aging through comparative analysis of molecular mechanisms via 5-HT2BR. Taken together, these results suggest that CaS has potential to improve wound closure and cell regeneration in human keratinocytes.
MATERIALS AND METHODS
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea (NRF) grants (NRF-2016R1D1A1B03930314, NRF-2016 R1D1A1B03931467, and NRF-2017R1C1B1003150) funded by the Korean government.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Kang K, Lee K, Ishihara A, et al. Induced synthesis of caffeoylserotonin in pepper fruits upon infection by the anthracnose fungus, Colletotrichum gloeosporioides. Sci Hortic. 2010;124:290–293. doi: 10.1016/j.scienta.2009.12.036. [DOI] [Google Scholar]
- 2.Choi J, Kim H, Choi Y, et al. Cytoprotective activities of hydorxycinnamic acid amides of serotonin against oxidative stress-induced damage in HepG2 and HaCaT cells. Fitoterapia. 2010;81:1134–1141. doi: 10.1016/j.fitote.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen C, Kim H, Lee S. Caffeoylserotonin protects human keratinocyte HaCaT Cells against H2O2-induced oxidative stress and apoptosis through upregulation of HO-1 expression via activation of the PI3K/Akt/Nrf2 Pathway. Phytother Res. 2013;27:1810–1818. doi: 10.1002/ptr.4931. [DOI] [PubMed] [Google Scholar]
- 4.Munz B, Frank S, Hübner G, Olsen E, Werner S. Novel type of glutathione peroxidase: expression and regulation during wound repair. Biochem J. 1997;326:579–585. doi: 10.1042/bj3260579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Toole E, Goel M, Woodley D. Hydrogen peroxide inhibits human keratinocyte migration. Dermatol Surg. 1996;22:525–529. doi: 10.1111/j.1524-4725.1996.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 6.Cerutti P, Trump B. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 7.James T, Hughes M, Cherry G, Taylor R. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen. 2003;11:172–176. doi: 10.1046/j.1524-475X.2003.11304.x. [DOI] [PubMed] [Google Scholar]
- 8.Musalmah M, Nizrana M, Fairuz A, et al. Comparative effects of palm vitamin e and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids. 2005;40:575–580. doi: 10.1007/s11745-005-1418-9. [DOI] [PubMed] [Google Scholar]
- 9.Senel O, Cetinkale O, Özbay G, Ahçioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. 1997;39:516–523. doi: 10.1097/00000637-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Masaki H. Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010;58:85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Nebigil C, Launay J, Hickel P, Toursnois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci U S A. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Zhang S, Li M, Hertz L, Peng L. Serotonin increases ERK1/2 phosphorylation in astrocytes by stimulation of 5-HT2B and 5-HT2C receptors. Neurochem Int. 2010;57:432–439. doi: 10.1016/j.neuint.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Pisarchik A, Abytek B, et al. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- 14.Draetta G, Luca F, Westendorf J, et al. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- 15.Ruhul Amin A, Senga T, Oo M, Thant A, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1β: a role for the dual signaling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwak S, Park B, Kim H, et al. Association of variations in TPH1 and HTR2B with gestational weight gain and measures of obesity. Obesity (Silver Spring) 2012;20:233–238. doi: 10.1038/oby.2011.253. [DOI] [PubMed] [Google Scholar]
- 17.Naito K, Tanaka C, Mitsuhashi M, et al. Signal Transduction mechanism of serotonin 5-HT2B receptormediated DNA synthesis and proliferation in primary culture of adult rat hepatocytes. Bol Pharm Bull. 2016;39:121–129. doi: 10.1248/bpb.b15-00735. [DOI] [PubMed] [Google Scholar]
- 18.Dai S, Yu L, Shi X, et al. Serotonin regulates osteoblast proliferation and function in vitro. Braz J Med Biol Res. 2014;47:759–765. doi: 10.1590/1414-431X20143565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang F, Lee J, Navolanic P, et al. Involvement of I3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 20.Alan Diehl J, Cheng M, Roussel M, Sherr C. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subvelular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Tian X, Mao G, et al. Peroxisome proliferatoractivated receptor-γ ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension. 2012;16:1471–1478. doi: 10.1161/HYPERTENSIONAHA.112.198887. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimkhani M, Oakley F, Murphy L, et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med. 2011;17:1668–1674. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.