Abstract
Background:
Many studies have highlighted sex preponderance in idiopathic pulmonary arterial hypertension (IPAH). It is well established that there are differences in exercise capacities in the two sexes but how much of that difference reflects on disease severity or correlates to markers of severity in the two sexes is still not clear. Right heart catheterization (RHC) and cardiopulmonary exercise testing (CPET) have been widely used for assessing functional capacity, prognosis and treatment response in IPAH. We aimed to investigate the ‘sex-specific’ CPET parameters in relation to hemodynamics in IPAH.
Methods:
Data were retrieved from 30 males and 53 females [mean ± standard deviation (SD) age: 39.6 ± 17.2 and 37.5 ± 12.0] stable IPAH patients who underwent both RHC and CPET at Shanghai Pulmonary Hospital from 2010 to 2016. Univariate and forward/backward multiple stepwise regression analysis was performed to assess the prognostic value of CPET and hemodynamic parameters.
Results:
There were no significant differences in clinical variables between men and women. Peak workload, peak oxygen uptake, anaerobic threshold (AT), peak minute ventilation, carbon dioxide output, O2 pulse and oxygen uptake efficiency slope were significantly higher in men compared with women (p < 0.05). Several CPET indexes correlated with hemodynamics. Pulmonary vascular resistance (PVR) and cardiac output (CO) were distinctly different between the sexes. Peak end-tidal partial pressure of CO2 (PETCO2) was an independent predictor of PVR elevation in all patients and in men. Peak maximum oxygen consumption (VO2) was independently predictive of CO decline in all patients and in men. Only peak O2 pulse was an independent predictor of increased PVR and decreased CO in women.
Conclusions:
Even after adjusting for age, body mass index and World Health Organization functional class, different CPET parameters correlated with PVR elevation and CO decline in men and women differently, which could potentially better predict severity in men and women with IPAH.
Keywords: CO, CPET, IPAH, PVR, sex difference
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a complex condition with multifactorial pathophysiology. While new developments regarding epidemiology and therapeutic options in IPAH are being reported [Hoeper et al. 2013; Humbert et al. 2010], one important question has emerged over the years: how does a sex difference in the incidence of this disease reflect disease severity? Identified as the earliest modifier in all the epidemiological studies, IPAH occurs predominantly in females [Badesch et al. 2010], with incidence of 1.9- to 10-fold higher, depending on a specific subtype and registry of patients [Pugh and Hemnes, 2010]. With regards prognosis, the influence of sex is reversed [Shapiro et al. 2012]. Both the French and the US-based registry [Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL)], characterized prognostic indicators and concluded that males have worse outcomes [Benza et al. 2010; Mathai et al. 2015]. In recent years, many studies have already demonstrated sex affects some signal pathways in the development of PAH [Kandhi et al. 2015; Mair et al. 2015; Wallace et al. 2015], although the majority of studies demonstrate effects of sex difference on cause, treatment and survival in PAH [Martin and Pabelick, 2014]. The hypothesis we propose is that sex differences exist in the prognostic assessment indexes of the same test method. However, to date, there is no clear consensus of whether sex difference has an impact on alteration of exercise capacity, metabolism, ventilation, cardiovascular and gas exchange parameters in patients with IPAH. Gas exchange measurements during cardiopulmonary exercise testing (CPET) must be looked at with regards to cellular respiration and how cardiopulmonary mechanisms are coupled to it. Most IPAH patients have gas exchange limitations attributed to an impaired vasodilator response of the pulmonary arteries to exercise [Tan et al. 2014]. CPET describes the underlying physiologic abnormalities associated with the hypoperfusion of the pulmonary vascular bed in IPAH [Oudiz et al. 2006], which has been used to gain insights into exertional abnormalities in patients with PAH for decades. Exercise limitations in PAH are the result of dysfunctions from various physiological systems and CPET has been used extensively for evaluating these dysfunctions. This is central to some recently published important reviews [Pinkstaff et al. 2016; Babu et al. 2016]. Usefulness of CPET owes to its ability to uncover abnormalities in the VO2 cascade [Sherry et al. 2016]. Additionally, CPEt allows reproducible assessment of functional capacity and treatment efficacy in patients with IPAH [Wensel, 2002]. There are massive differences in CPET parameters between healthy male and female subjects, but whether these differences are replicated in patients with IPAH is still unclear. In addition, whether different CPET parameters can predict outcomes in male and female patients with IPAH is also unknown.
Thus, the objectives of the present study were to explore whether sex difference has an impact on alteration of CPET parameters in patients with incident IPAH and to correlate CPET measurements with hemodynamic variables as measured by right-heart catheterization (RHC).
Methods
Population study
From May 2010 to February 2016, 83 patients (30 males, 53 females) with incident IPAH, aged more than 18-years old, were evaluated at Shanghai Pulmonary Hospital, Shanghai. The diagnosis of IPAH was established according to the most recent pulmonary hypertension guidelines, specifically, by the presence of a mean pulmonary arterial pressure (mPAP) > 25 mmHg and a pulmonary capillary wedge pressure (PCWP) ⩽ 15 mm Hg, as assessed by RHC [Hoeper et al. 2013]. Patients with PAH associated with a definite cause, such as connective tissue disease and congenital heart disease, and those with portopulmonary hypertension, chronic pulmonary obstruction, chronic pulmonary thromboembolism and pulmonary hypertension due to left heart disease were excluded. We also excluded patients with acute or chronic illnesses that might influence hormonal metabolism (i.e. acute or chronic infections, chronic autoimmune diseases, previously established primary endocrine disorders), and patients receiving any hormonal treatment (thyroid hormones, anabolic steroids, corticosteroids) or drugs that markedly inhibit hormone production, either at the time of the study or in the past.
The study protocol was reviewed and approved by the Ethics Committee of Shanghai Pulmonary Hospital (Ethics Approval No.: K12-089). Written informed consent was obtained from each patient for inclusion into the study and prior to the performance of any study-related procedures.
Assessment of patients
Patients underwent RHC and a 6-minute walk distance (6MWD) test within 3 months of each patient’s CPET study, their World Health Organization functional class (WHO FC) and N-terminal Natriuretic peptide type-B (NT-proBNP) were determined. RHC was performed following standard protocols [Jing et al. 2011]. The 6MWD test was performed according to American Thoracic Society guidelines [American Thoracic Society, 2002], and a Borg dyspnea score was determined immediately after the 6MWD test. Immediately prior to their CPET studies, patients had standard pulmonary function tests.
Each patient performed a physician-supervised, standard, progressively increasing work rate CPET to maximum tolerance on an electromagnetically braked cycle ergometer. The protocol comprised of 3 minutes of rest, 3 minutes of unloaded cycling at 55–65 revolutions per minute (rpm), followed by a progressively increasing work rate of 5–15 Watts (W)/minute for PH patients and 20–25 W/minute for the normal subjects to the maximum tolerance, and 4 minutes of recovery [Sue and Wasserman, 1991]. Pulse oximetry, heart rate (HR), 12-lead electrocardiography, and cuff blood pressure were monitored and recorded. Minute ventilation (VE, BTPS), maximum oxygen consumption (VO2, STPD), carbon dioxide output (VCO2, STPD), and other exercise variables were computer calculated breath by breath, interpolated second by second, and averaged over 10-second intervals [Sue and Wasserman, 1991]. The anaerobic threshold (AT) ratio of oxygen pulse (O2 pulse) was determined as previously described [Magalang and Grant, 1995]. Exercise, metabolic and cardiovascular capacity were expressed as workload, VO2, O2 pulse and HR respectively. Ventilatory efficiency during exercise was expressed as VCO2, VE, the ratio of VO2/VE, VE/VCO2 (oxygen uptake efficiency plateau) OUEP, oxygen uptake efficiency slope (OUES) and the slope of VE versus VCO2 over the linear component of the plot of VE versus VCO2 [Shi et al. 2016]. Gas exchange efficiency was expressed as carbon dioxide elimination/oxygen consumption (RER) and end-tidal partial pressure of CO2 (PETCO2). The rate of VO2 increase during unloaded cycling was expressed as the mean response time (MRT) for a monoexponential curve fit to the second-by-second VO2 measurements during the 3 minutes of unloaded cycling [Sun et al. 2001]. If the first breath VO2 equaled the 3-minute VO2, the MRT was considered equal to the duration of the first breath.
Statistical analysis
Results are expressed as mean with standard deviation (SD) or medians (and interquartile range) for continuous variables and absolute number for categorical variables. The sample size and the power were analyzed by NCSS-PASS (© Copyright 2011 NCSS LLC., Kaysville, UT) version 11.0.7. Comparisons were performed using Student’s t test or Mann–Whitney U test, for continuous variables and chi-square test for categorical variables. Correlations were assessed using Spearman’s rank correlation coefficient. To determine the strength of the association found between hemodynamic and CPET variables found to correlate with IPAH at univariate analysis, we performed forward/backward multiple stepwise regression analysis with hemodynamic variables as the dependent outcome. We forced age, body mass index (BMI) and WHO FC into the models to adjust multiple regression analysis. p values < 0.05 were considered significant. The main analysis was performed using SPSS (Statistic Package for Social Science, Chicago, IL) version 19.0.
Results
Patient population
Demographics, baseline and hemodynamic data are illustrated in Table 1. There was no difference in BMI between the two sexes and age was almost matched with mean age of 39.6 ± 17.2 years in males and 37.5 ± 12.0 years in females. A total of 83 patients had WHO FC reported at diagnosis. Yet there were no differences in overall WHO FC between the two groups. Men and women had similar absolute 6MWD and NT pro-BNP levels and mean right-arterial pressure (mRAP), mPAP, mean pulmonary capillary wedge pressure (mPAWP), pulmonary vascular resistance (PVR), CO and mixed venous O2 saturation at diagnosis were also similar between the two sexes. The differences of the treatment between the two sexes were not found either.
Table 1.
Baseline characteristics of all patients with idiopathic pulmonary arterial hypertension.
| All (n = 83) |
Men (n = 30) |
Women (n = 53) |
p value | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, y | 38. ± 14.0 | 39.6 ± 17.2 | 37.5 ± 12.0 | 0.547 |
| BMI, kg/m2 | 22.6 ± 3.1 | 23.0 ± 3.2 | 22.4 ± 3.0 | 0.457 |
| WHO-FC, n (%) | 0.494 | |||
| I–II | 48 (57.8) | 19 (63.3) | 29 (54.7) | |
| III–IV | 35 (42.2) | 11 (36.7) | 24 (45.3) | |
| 6MWD, m | 399.0 ± 118.2 | 406.6 ± 125.4 | 394.4 ± 115.0 | 0.689 |
| NT pro-BNP, pg/ml | 334 (167, 1096) | 186 (23, 1096) | 524 (155, 1226) | 0.395 |
| Hemodynamics | ||||
| mRAP, mmHg | 5.7 ± 4.3 | 6.4 ± 5.0 | 5.3 ± 3.7 | 0.270 |
| mPAP, mmHg | 58.1 ± 13.8 | 57.7 ± 16.0 | 58.3 ± 12.5 | 0.867 |
| mPAWP, mmHg | 7.2 ± 3.4 | 7.6 ± 2.2 | 6.9 ± 4.0 | 0.320 |
| PVR, Wood units | 13.0 ± 5.7 | 11.9 ± 5.6 | 13.5 ± 5.7 | 0.222 |
| CO, l/min | 4.6 ± 1.6 | 4.8 ± 1.8 | 4.4 ± 1.4 | 0.375 |
| SVO2, % | 64.8 ± 10.1 | 63.2 ± 10.2 | 66.0 ± 10.0 | 0.310 |
| Treatment | 0.618 | |||
| Specific medications | ||||
| PDE-5 inhibitors, % | 35 (42.2) | 12 (40.0) | 23 (43.4) | |
| ERAs, % | 15 (18.1) | 4 (13.3) | 11 (20.8) | |
| Prostacyclin analog, % | 4 (4.8) | 1 (3.3) | 3 (5.7) | |
| Combination, % | 22 (26.5) | 11 (36.7) | 11 (20.8) | |
| No specific medication | 4 (4.8) | 2 (6.7) | 2 (3.8) |
Values are mean (SD) or median (interquartile range).
6MWD, 6-minute walk distance; BMI, body mass index; CO, cardiac output; ERAs, endothelial receptor antagonists; mPAP, mean pulmonary arterial pressure; mPAWP, mean pulmonary capillary wedge pressure; mRAP, mean right arterial pressure; NT pro-BNP, N-Terminal pro-brain natriuretic peptide; PDE-5, phosphodiesterase type 5; PVR, pulmonary vascular resistance; SVO2, mixed venous O2 saturation; WHO-FC, World Health Organization functional class.
Sex differences of cardiopulmonary exercise testing parameters in patients with idiopathic pulmonary arterial hypertension
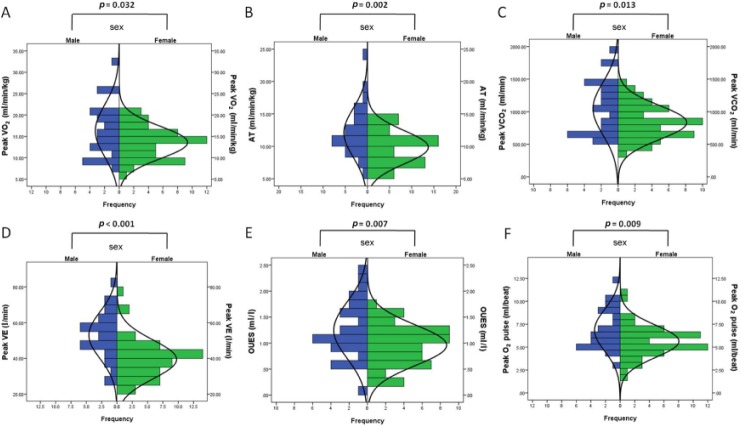
CPET results are presented in Table 2. Peak workload and the metabolic parameter, that is, peak VO2 and AT was significantly higher in male patients with IPAH. Male patients also had higher peak VE and peak VCO2. OUES, representing gas exchange indices, was also higher in male IPAH patients than females. Peak O2 pulse, one of the cardiovascular parameters, also showed significantly higher levels in males than females. OUEP, VE versus VCO2 slope, peak HR, peak RER, peak PETCO2 and PETCO2 at AT were not significantly different between male and female IPAH patients. Figure 1 contrasts key CPET measurement differences and frequencies between the two groups.
Table 2.
Cardiopulmonary exercise testing parameters between male and female patients with idiopathic pulmonary arterial hypertension.
| Variables | All (n = 83) |
Men (n = 30) |
Women (n = 53) |
p value |
|---|---|---|---|---|
| Exercise capacity parameters | ||||
| Peak workload, Watts | 72.4 ± 29.4 | 82.0 ± 36.1 | 67.0 ± 23.6 | 0.046 |
| Metabolic parameters | ||||
| Peak VO2, ml/min/kg | 14.6 ± 4.9 | 16.3 ± 6.1 | 13.6 ± 3.8 | 0.032 |
| AT, ml/min/kg | 10.7 ± 3.2 | 12.1 ± 3.8 | 9.9 ± 2.6 | 0.002 |
| Ventilatory parameters | ||||
| Peak VE, l/min | 44.4 ± 13.3 | 53.0 ± 13.1 | 39.5 ± 10.9 | <0.001 |
| Peak VCO2, ml/min | 910.5 ± 333.1 | 1046.2 ± 403.0 | 833.5 ± 260.2 | 0.013 |
| OUES, l/min/log | 1.1 ± 0.5 | 1.2 ± 0.5 | 1.0 ± 0.4 | 0.007 |
| OUEP, ml/l | 27.4 ± 7.2 | 27.9 ± 9.8 | 27.1 ± 5.2 | 0.645 |
| VE/VCO2 slope | 52.4 ± 26.9 | 58.8 ± 37.9 | 48.8 ± 17.4 | 0.102 |
| Cardiovascular parameters | ||||
| Peak O2 pulse, ml/beat | 6.1 ± 2.0 | 6.8± 2.3 | 5.6 ± 1.7 | 0.009 |
| Peak HR, beats/min | 142.4 ± 24.7 | 141.7 ± 28.1 | 142.7 ± 22.8 | 0.850 |
| Gas exchange parameters | ||||
| Peak RER | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.443 |
| Peak PETCO2, mmHg | 25.4 ± 6.8 | 24.4 ± 7.4 | 26.0 ± 6.3 | 0.316 |
| PETCO2 at AT, mmHg | 28.0 ± 6.8 | 27.0 ± 7.3 | 28.6 ± 6.4 | 0.305 |
Values are mean (SD).
AT, anaerobic threshold; IPAH, idiopathic pulmonary arterial hypertension; OUEP, oxygen uptake efficiency plateau; OUES, oxygen uptake efficiency slope; PETCO2, end-tidal partial pressure of CO2; PVR, pulmonary vascular resistance; RER, carbon dioxide elimination/oxygen consumption; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
Figure 1.
Cardiopulmonary exercise testing parameters in male and female idiopathic pulmonary arterial hypertension patients.
AT, anaerobic threshold; OUES, oxygen uptake efficiency slope; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
Relationship between cardiopulmonary exercise testing and hemodynamic parameters in patients with idiopathic pulmonary arterial hypertension
Correlations between CPET parameters and hemodynamics are illustrated in Tables 3 and 4. Peak workload, peak VO2, AT, peak VCO2, OUES, peak O2 pulse, peak PETCO2 and PETCO2 at AT had significant negative correlations with PVR in all IPAH patients. Peak VO2, peak VCO2, OUES, peak O2 pulse, peak PETCO2 and PETCO2 at AT were negatively correlated to PVR in males, while only peak O2 pulse negatively correlated with PVR in females only. Only VE/VCO2 slope had significant positive correlation with PVR in all IPAH patients as a whole or in male patients when comparing them as three separate groups.
Table 3.
Correlations of cardiopulmonary exercise testing parameters to pulmonary vascular resistance in patients with idiopathic pulmonary arterial hypertension.
| Variables | All (n = 83) |
Men (n = 30) |
Women (n = 53) |
|||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| Exercise capacity parameters | ||||||
| Peak workload, Watts | −0.133 | 0.233 | −0.150 | 0.430 | −0.068 | 0.631 |
| Metabolic parameters | ||||||
| Peak VO2, ml/min/kg | −0.289 | 0.009 | −0.329 | 0.006 | −0.222 | 0.114 |
| AT, ml/min/kg | −0.186 | 0.095 | −0.192 | 0.308 | −0.121 | 0.394 |
| Ventilatory parameters | ||||||
| Peak VE, l/min | 0.004 | 0.973 | 0.173 | 0.361 | 0.023 | 0.871 |
| Peak VCO2, ml/min | −0.290 | 0.008 | −0.266 | 0.015 | −0.273 | 0.051 |
| OUES, l/min/log | −0.290 | 0.009 | −0.429 | 0.018 | −0.149 | 0.301 |
| OUEP, ml/l | −0.094 | 0.402 | 0.152 | 0.438 | −0.352 | 0.051 |
| VE/VCO2 slope | 0.260 | 0.018 | 0.352 | 0.044 | 0.270 | 0.053 |
| Cardiovascular parameters | ||||||
| Peak O2 pulse, ml/beat | −0.386 | <0.001 | −0.336 | 0.009 | −0.393 | 0.004 |
| Peak HR, beats/min | 0.141 | 0.207 | 0.127 | 0.504 | 0.153 | 0.279 |
| Gas exchange parameters | ||||||
| Peak RER | 0.105 | 0.361 | 0.107 | 0.595 | 0.131 | 0.359 |
| Peak PETCO2, mmHg | −0.349 | 0.001 | −0.445 | 0.014 | −0.324 | 0.059 |
| PETCO2 at AT, mmHg | −0.300 | 0.006 | −0.340 | 0.016 | −0.308 | 0.056 |
Data are Spearman’s rank correlation coefficient or Pearson.
AT, anaerobic threshold; OUEP, oxygen uptake efficiency plateau; OUES, oxygen uptake efficiency slope; PETCO2, end-tidal partial pressure of CO2; PVR, pulmonary vascular resistance; VCO2, carbon dioxide output; VE, minute ventilation; VO2, maximum oxygen consumption; HR, heart rate; RER, carbon dioxide elimination/oxygen consumption.
Table 4.
Correlations of cardiopulmonary exercise testing parameters to cardiac output in patients with idiopathic pulmonary arterial hypertension.
| Variables | All (n = 83) |
Men (n = 30) |
Women (n = 53) |
|||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| Exercise capacity parameters | ||||||
| Peak workload, Watts | 0.238 | 0.032 | 0.312 | 0.094 | 0.121 | 0.391 |
| Metabolic parameters | ||||||
| Peak VO2, ml/min/kg | 0.433 | <0.001 | 0.612 | <0.001 | 0.209 | 0.137 |
| AT, ml/min/kg | 0.318 | 0.004 | 0.474 | 0.008 | 0.126 | 0.373 |
| Ventilatory parameters | ||||||
| Peak VE, l/min | 0.287 | 0.059 | 0.037 | 0.847 | 0.482 | 0.050 |
| Peak VCO2, ml/min | 0.444 | <0.001 | 0.432 | 0.017 | 0.447 | 0.001 |
| OUES, l/min/log | 0.372 | 0.001 | 0.448 | 0.013 | 0.280 | 0.051 |
| OUEP, ml/l | 0.170 | 0.128 | 0.213 | 0.258 | 0.110 | 0.480 |
| VE/VCO2 slope | −0.188 | 0.045 | −0.278 | 0.137 | −0.122 | 0.388 |
| Cardiovascular parameters | ||||||
| Peak O2 pulse, ml/beat | 0.379 | <0.001 | 0.341 | 0.065 | 0.393 | 0.001 |
| Peak HR, beats/min | −0.076 | 0.500 | 0.141 | 0.457 | −0.021 | 0.881 |
| Gas exchange parameters | ||||||
| Peak RER | −0.055 | 0.634 | −0.046 | 0.820 | −0.078 | 0.588 |
| Peak PETCO2, mmHg | 0.199 | 0.033 | 0.441 | 0.015 | 0.020 | 0.890 |
| PETCO2 at AT, mmHg | 0.196 | 0.037 | 0.391 | 0.032 | 0.061 | 0.666 |
Data are Spearman’s rank correlation coefficient or Pearson.
AT, anaerobic threshold; OUEP, oxygen uptake efficiency plateau; OUES, oxygen uptake efficiency slope; PETCO2, end-tidal partial pressure of CO2; VCO2, carbon dioxide output; VE, minute ventilation; VO2, maximum oxygen consumption; HR, heart rate; RER, carbon dioxide elimination/oxygen consumption.
Peak workload, peak VO2, AT, peak VCO2, OUES, peak O2 pulse, peak PETCO2 and PETCO2 at AT correlated positively with CO in all patients. Peak VO2, AT, peak VCO2, OUES, peak PETCO2 and PETCO2 at AT correlated positively with CO in male IPAH patients. Meanwhile, peak VCO2 and peak O2 pulse correlated positively with CO in female group. Only VE/VCO2 slope had significant negative correlation with CO in all IPAH patients.
Factors influencing pulmonary ventilation rate and cardiac output
Significantly correlated variables were entered into stepwise multiple regression analyses to determine the strength of each individual parameter to predict PVR elevation. As shown in Table 5, for the whole 83 IPAH patients, the model 1 adjusted by age, gender, BMI and WHO FC, peak PETCO2 was an independent predictor and explained 12.2% (R2 = 0.122) of the variation in this measurement. In male IPAH patients, model 2 adjusted by age, BMI and WHO FC, peak PETCO2 was the independent predictor accounting for 19.8% (R2 = 0.198) of the variation. In the females, model 2, peak O2 pulse was the sole independent predictor of PVR, separately accounting for 15.5% (R2 = 0.155) of the variation.
Table 5.
Determinants of pulmonary vascular resistance and cardiac output in all idiopathic pulmonary arterial hypertension patients.
| Populations studied | Model | Independent variables | R 2 | Standardized β | 95% CI | p value |
|---|---|---|---|---|---|---|
| Dependent variables: PVR | ||||||
| All patients (n = 83) |
Model 1 | Peak PETCO2 | 0.122 | −0.291 | −0.465~−0.117 | 0.001 |
| Male patients (n = 30) |
Model 2 | Peak PETCO2 | 0.198 | −0.33 | −0.592~−0.073 | 0.014 |
| Female patients (n = 53) |
Model 2 | Peak O2 pulse | 0.155 | −1.271 | −2.115~−0.428 | 0.004 |
| Dependent variables: CO | ||||||
| All patients (n = 83) |
Model 1 | Peak VO2 | 0.187 | 0.138 | 0.074~0.202 | <0.001 |
| Male patients (n = 30) |
Model 2 | Peak VO2 | 0.375 | 0.181 | 0.091~0.272 | <0.001 |
| Female patients (n = 53) |
Model 2 | Peak O2 pulse | 0.155 | 0.317 | 0.106~0.527 | 0.004 |
Model 1, adjust for age, sex, BMI and WHO FC; Model 2, adjust for age, BMI and WHO FC;
CI, confidence interval; PETCO2, end-tidal partial pressure of CO2; VO2, maximum oxygen consumption, PVR, pulmonary vascular resistance; CO cardiac output.
Peak VO2 was the sole independent predictor of CO decline and accounted for 18.7% (R2 = 0.187) of the variations in this measurement for all IPAH patients (associated model 1) and 37.5% (R2 = 0.375) in male patients (associated model 2). In female patients, model 2, peak O2 pulse was the sole independent predictor of CO accounting for 15.5% (R2 = 0.155) of the variation.
Discussion
CPET’s increasing credibility is reflected by the fact that major guidelines for the treatment and management of PAH have endorsed it [Galiè et al. 2016]. Evidence since the last 2 decades and beyond have already established that there are differences in exercise capacities between the two sexes [Astrand, 1956; Astrand et al. 1973; Bruce et al. 1973]. Also, work efficiency in humans is relatively fixed for a given work task as well. While race, body build and genetic predispositions can all influence exercise capacity, how a differently reduced exercise capacity between the two sexes reflects impaired hemodynamics is still not clear [Wasserman and Whipp, 1975]. It has been well established that VO2/kg fairly represents metabolic demand. Both peak VO2 and VE/VCO2 slope and their predicted normal for a particular patient would be fairly accurate in terms of their predictive ability [McLaughlin et al. 2013; Schwaiblmair et al. 2012]. The current paper perhaps is the first to report that a relationship between CPET parameters and PVR as well as CO exists in IPAH. Sex differences of CPET parameters have received far less attention than hemodynamic parameters in IPAH. We hope this paper will serve its purpose of trying to reason why the difference in exercise capacities affects hemodynamics in a particular disease spectrum, in this case, IPAH. Although being an initial small sample study (n = 83), several CPET indexes reflected ‘sex-specific’ differences and some of them were able to predict PVR and CO independently.
There were no significant sex differences in WHO-FC, 6MWD, NT-proBNP or hemodynamics at the time of diagnosis and treatment in our study, but higher trends of mPAP, mRAP, CO and 6MWD in men were visible, implying that men with IPAH have relatively lower pulmonary vascular function, yet higher cardiovascular function and exercise ability. Thus, sex may affect the hemodynamics in our study. The REVEAL Registry indicated that male PAH patients categorized as New York Heart Association Functional Classification I-II (NYHA FCI-II), had higher mPAP, mRAP, CO and 6MWD at diagnosis [Shapiro et al. 2012].Their results are not completely consistent with ours. One possible reason is our small sample size, while the REVEAL Registry included 2969 patients, which is a large sample study. Another possible reason is that they classified patients differently, that is, patients in our study labeled as IPAH, are classified as IPAH, FPAH, CTD-PAH, CHD-PAH and other associated PAH subgroups in the REVEAL Registry [Shapiro et al. 2012].
The actual values for all CPET variables were compared between the two sexes because predicted values are often adjusted for some factors, that is, sex. Jarvis and colleagues demonstrated that differences in muscle mass, fiber type or the way energy production takes place in the two sexes in healthy humans, suggesting male IPAH patients demonstrate higher metabolism and ventilation compared with female patients [Jarvis et al. 2011]. Our data indicate that exercise capacity, metabolic, ventilatory and cardiovascular parameters, the four key aspects of any CPET, were all higher in male IPAH patients than in females. As described above, perhaps a larger muscle mass, as seen in males, is a reason that male patients probably have better cardiovascular and exercise pliability. The role of sex hormones in causation of IPAH may be another key factor as well when interpreting these sex differences. Sex hormones, especially estradiol and its metabolites, may affect metabolic enzymes, some signal pathway and epigenetics in cardiopulmonary vascular systems [Kandhi et al. 2015; Mair et al. 2015; Wallace et al. 2015]. In either case, severe limitations are seen with cardiopulmonary function in female patients with IPAH.
CPET with gas exchange measurements has the potential of noninvasively grading the severity of exercise limitation, quantifying the hypoperfusion of the lung and systemic circulation, and assessing responses to treatment before overt right ventricular failure, and PAH is evident even at rest [Wax et al. 1999; Wensel et al. 2000]. Pinkstaff and colleagues go even further by making class recommendations for CPET, for diagnostic evaluation (Level B, Class IIa), prognostication (Level B, class IIb) and determining therapeutic efficiency (Level C, class IIb) [Pinkstaff et al. 2016].
The continued PVR elevation in IPAH due to progressive pulmonary vasculopathy, limits blood flow through the lung and thus through the body, thereby impairing aerobic regeneration of ATP. Therefore, more work is done anaerobically at relatively low workloads and decreased cardiovascular reserve. Thus, a wide range of correlations between CPET parameters, and PVR and CO were seen in all patients in the present study. Not only due to sex difference, different body structures, or because PVR is the product of mPAP and CO, but also because men may have higher baseline mPAP, mRAP and CO [Lahm et al. 2014; Shapiro et al. 2012].
Along with gas exchange inefficiency and metabolic abnormalities, exercise and cardiovascular function are also impaired, which contribute to a decline in CO in IPAH [Guo et al. 2015; Tan et al. 2014]. Peak VO2, as a metabolic parameter, assesses the subject’s maximal exercise ability and the maximal compensatory ability of the circulatory system to raise CO [Sun et al. 2001]. The decreased PETCO2 in IPAH or cardiac failure patients is considered derived from the ventilation–perfusion mismatch and the increased ratio of physiologic dead space to tidal volume [Matsumoto et al. 2000; Yasunobu et al. 2005]. Peak O2 pulse, as a cardiovascular parameter, likely reflects a progressive reduction in peak stroke volume paralleling disease severity with its progressive decline [Sun et al. 2001]. Although the number of female patients was 1.33 times the number of male patients in the present study, the multivariate analyses of all patients showed similar results to that of men, suggesting that all patients perhaps have more severe gas exchange inefficiency and metabolic abnormalities than cardiovascular abnormalities. Multivariate analyses also indicated that peak PETCO2 and VO2 were associated most with PVR elevation and CO decline in male patients with IPAH. These results demonstrate that men may have worse gas exchange efficiency and metabolic abnormalities. Peak O2 pulse can be considered as independent predictor of PVR elevation and CO decline in female patients, suggesting women may have worse cardiovascular dysfunction. Interestingly, peak PETCO2 was an independent predictor of increased PVR in all patients, while peak VO2 was also an independent predictor of CO decline in such patients. However, the exact reason why men and women had different results on multivariate analyses needs to be further explored, and whether sex hormones contribute to this also needs further research.
IPAH patients included in this study were small, and without further PAH subgroup classification, that is, FPAH, CTD-PAH, CHD-PAH. Therefore, the sex differences of CPET variables are valid only specifically for IPAH patients for now. Because CPET variables may be abnormal in patients with other PAH subgroups, the independent predictors of increased PVR and decreased CO perhaps could be reflected by other CPET variables in those disease groups as well. In addition, other factors, that is, race and genetic predisposition, could influence metabolic, ventilatory and exercise capacity, but were not considered in the present study.
In summary, our findings suggest that several CPET indexes showed ‘sex-specific’ differences and some of them could also predict PVR and CO independently. Further validation of these sex differences are needed from larger samples of PAH patients to determine if sex hormones contribute to the difference in CPET variables.
Acknowledgments
All authors actively participated in the study and in the review and approval of the manuscript. PY, J-ML and Q-HH contributed to drafting of the manuscript and to study concept and design; PY and T-XC contributed to data acquisition; PY, BP, S-JZ, Q-HZ, LW, RJ, W-HW and JH contributed to data analysis and interpretation; BP, JZ, JG, LW, S-GG and J-ML contributed to critical revision of the manuscript for important intellectual content; PY, T-XC and J-ML contributed to statistical analysis; J-ML and Q-HH contributed to study supervision; PY and J-ML contributed to acquisition of funding. PY, T-XC, BP and JZ contributed equally to this article.
Footnotes
Funding: This work was supported by the Program of Shanghai Municipal Commission of Health and Family Planning [grant number 20144Y0196], Shanghai Natural Science Foundation [grant number 15ZR1434400] and National China Natural Science Foundation [grant number 81500040].
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ping Yuan, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Tian-Xiang Chen, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Bigyan Pudasaini, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Jie Zhang, The Organization and Personnel Department, Qilu Children’s Hospital of Shandong University, Shandong University, Shandong, China.
Jian Guo, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Si-Jin Zhang, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Lan Wang, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Qin-Hua Zhao, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Su-Gang Gong, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Rong Jiang, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Wen-Hui Wu, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Jing He, Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, Shanghai, China.
Jin-Ming Liu, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, No. 507 Zhengmin Road, Shanghai 200433, China.
Qing-Hua Hu, Shanghai Pulmonary Hospital, Tongji University, School of Medicine, No. 507 Zhengmin Road, Shanghai 200433, China.
References
- American Thoracic Society. (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117. [DOI] [PubMed] [Google Scholar]
- Astrand P. (1956) Human physical fitness with special reference to sex and age. Physiol Rev 36: 307–335. [DOI] [PubMed] [Google Scholar]
- Astrand I., Astrand P., Hallbäck I., Kilbom A. (1973) Reduction in maximal oxygen uptake with age. J Appl Physiol 35: 649–654. [DOI] [PubMed] [Google Scholar]
- Babu A., Arena R., Myers J., Padmakumar R., Maiya A., Cahalin L., et al. (2016) Exercise intolerance in pulmonary hypertension: mechanism, evaluation and clinical implications. Expert Rev Respir Med 10: 979–990. [DOI] [PubMed] [Google Scholar]
- Badesch D., Raskob G., Elliott C., Krichman A., Farber H., Frost A., et al. (2010) Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137: 376–387. [DOI] [PubMed] [Google Scholar]
- Benza R., Miller D., Gomberg-Maitland M., Frantz R., Foreman A., Coffey C., et al. (2010) Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172. [DOI] [PubMed] [Google Scholar]
- Bruce R., Kusumi F., Hosmer D. (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85: 546–562. [DOI] [PubMed] [Google Scholar]
- Galiè N., Humbert M., Vachiery J., Gibbs S., Lang I., Torbicki A., et al. (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119. [DOI] [PubMed] [Google Scholar]
- Guo J., Shi X., Yang W., Gong S., Zhao Q., Wang L., et al. (2015) Exercise physiology and pulmonaryhemodynamic abnormality in PH patients with exercise induced venous-to-systemic shunt. PloS One 10: e0121690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeper M., Bogaard H., Condliffe R., Frantz R., Khanna D., Kurzyna M., et al. (2013) Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- Humbert M., Sitbon O., Chaouat A., Bertocchi M., Habib G., Gressin V., et al. (2010) Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163. [DOI] [PubMed] [Google Scholar]
- Jarvis S., VanGundy T., Galbreath M., Shibata S., Okazaki K., Reelick M., et al. (2011) Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z., Yu Z., Shen J., Wu B., Xu K., Zhu X., et al. (2011) Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 183: 1723–1729. [DOI] [PubMed] [Google Scholar]
- Kandhi S., Qin J., Froogh G., Jiang H., Luo M., Wolin M., et al. (2015) EET-dependent potentiation of pulmonary arterial pressure: sex-different regulation of soluble epoxide hydrolase. Am J Physiol Lung Cell Mol Physiol 309: L1478–L1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T., Tuder R., Petrache I. (2014) Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L7–L26. [DOI] [PubMed] [Google Scholar]
- Magalang U., Grant B. (1995) Determination of gas exchange threshold by nonparametric regression. Am J Respir Crit Care Med 151(1): 98–106. [DOI] [PubMed] [Google Scholar]
- Mair K., Long L., White K., Wallace E., Ewart M., Docherty C., et al. (2015) Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 191: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Y., Pabelick C. (2014) Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H1253–H1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai S., Hassoun P., Puhan M., Zhou Y., Wise R. (2015) Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest 147: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Itoh H., Eto Y., Kato M., Omata M., Watanabe H., et al. (2000) End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol 36: 242–249. [DOI] [PubMed] [Google Scholar]
- McLaughlin V., Gaine S., Howard L., Leuchte H., Mathier M., Mehta S., et al. (2013) Treatment goals of pulmonary hypertension. J Am Coll Cardiol 62: D73–D81. [DOI] [PubMed] [Google Scholar]
- Oudiz R., Barst R., Hansen J., Sun X., Garofano R., Wu X., et al. (2006) Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol 97: 123–126. [DOI] [PubMed] [Google Scholar]
- Pinkstaff S., Burger C., Daugherty J., Bond S., Arena R. (2016) Cardiopulmonary exercise testing in patients with pulmonary hypertension: clinical recommendations based on a review of the evidence. Expert Rev Respir Med 10: 279–295. [DOI] [PubMed] [Google Scholar]
- Pugh M., Hemnes A. (2010) Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease. Womens Health (Lond) 6: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiblmair M., Faul C., von Scheidt W., Berghaus T. (2012) Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm Med 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Traiger G., Turner M., McGoon M., Wason P., Barst R. (2012) Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 141: 363–373. [DOI] [PubMed] [Google Scholar]
- Shi X., Guo J., Gong S., Sapkota R., Yang W., Liu H., et al. (2016) Oxygen uptake is more efficient in idiopathic pulmonary arterial hypertension than in chronic thromboembolic pulmonary hypertension. Respirology 2: 149–156. [DOI] [PubMed] [Google Scholar]
- Sue D., Wasserman K. (1991) Impact of integrative cardiopulmonary exercise testing on clinical decision making. Chest 99: 981–992. [DOI] [PubMed] [Google Scholar]
- Sun X., Hansen J., Oudiz R., Wasserman K. (2001) Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 104: 429–435. [DOI] [PubMed] [Google Scholar]
- Tan X., Yang W., Guo J., Zhang Y., Wu C., Sapkota R., et al. (2014) Usefulness of decrease in oxygen uptake efficiency to identify gas exchange abnormality in patients with idiopathic pulmonary arterial hypertension. PLoS One 9: e98889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace E., Morrell N., Yang X., Long L., Stevens H., Nilsen M., et al. (2015) A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med 191: 1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. (1975) Exercise physiology in health and disease. Am Rev Respir Dis 112: 219–249. [DOI] [PubMed] [Google Scholar]
- Wax D., Garofano R., Barst R. (1999) Effects of long-term infusion of prostacyclinon exercise performance in patients with primary pulmonary hypertension. Chest 116: 914–920. [DOI] [PubMed] [Google Scholar]
- Wensel R. (2002) Assessment of survival in patients with primary pulmonary hypertension: Importance of cardiopulmonary exercise testing. Circulation 106: 319–324. [DOI] [PubMed] [Google Scholar]
- Wensel R., Opitz C., Ewert R., Bruch L., Kleber F. (2000) Effects of iloprost inhalation on exercise capacity and ventilatory efficiency in patients with primary pulmonary hypertension. Circulation 101: 2388–2392. [DOI] [PubMed] [Google Scholar]
- Yasunobu Y., Oudiz R., Sun X., Hansen J., Wasserman K. (2005) End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 127: 1637–1646. [DOI] [PubMed] [Google Scholar]