Figure 2.
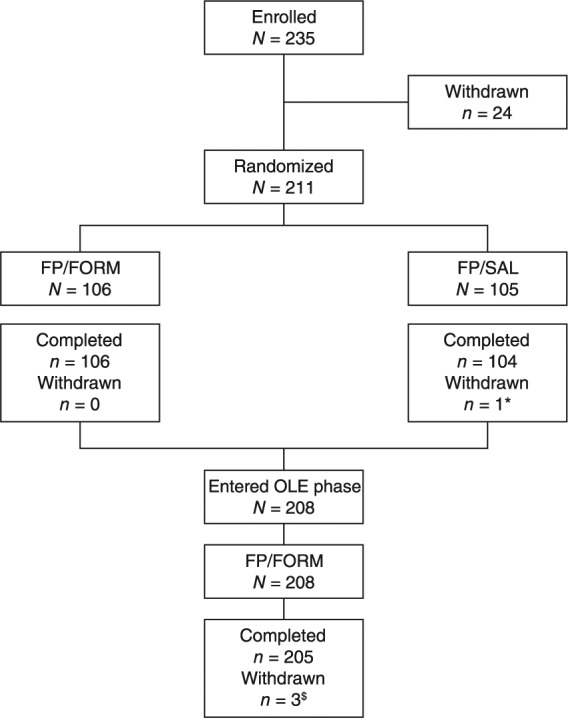
Patient disposition during the core trial and extension phase.
FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate; OLE, open-label extension phase; *patient withdrew by choice; $two patients withdrew by choice; one patient withdrew for administrative reasons.
