Abstract
Reactive oxygen species are well known for induction of oxidative stress conditions through oxidation of vital biomarkers leading to cellular death via apoptosis and other process, thereby causing devastative effects on the host organs. This effect is believed to be linked with pathological alterations seen in several neurodegenerative disease conditions. Many phytochemical compounds proved to have robust antioxidant activities that deterred cells against cytotoxic stress environment, thus protect apoptotic cell death. In view of that we studied the potential of glucomoringin-isothiocyanate (GMG-ITC) or moringin to mitigate the process that lead to neurodegeneration in various ways. Neuroprotective effect of GMG-ITC was performed on retinoic acid (RA) induced differentiated neuroblastoma cells (SHSY5Y) via cell viability assay, flow cytometry analysis and fluorescence microscopy by means of acridine orange and propidium iodide double staining, to evaluate the anti-apoptotic activity and morphology conservation ability of the compound. Additionally, neurite surface integrity and ultrastructural analysis were carried out by means of scanning and transmission electron microscopy to assess the orientation of surface and internal features of the treated neuronal cells. GMG-ITC pre-treated neuron cells showed significant resistance to H2O2-induced apoptotic cell death, revealing high level of protection by the compound. Increase of intracellular oxidative stress induced by H2O2 was mitigated by GMG-ITC. Thus, pre-treatment with the compound conferred significant protection to cytoskeleton and cytoplasmic inclusion coupled with conservation of surface morphological features and general integrity of neuronal cells. Therefore, the collective findings in the presence study indicated the potentials of GMG-ITC to protect the integrity of neuron cells against induced oxidative-stress related cytotoxic processes, the hallmark of neurodegenerative diseases.
Introduction
Reactive oxygen species (ROS) including hydrogen peroxide (H2O2) are known by their induction of oxidative stress believed to be linked with various neurodegenerative disease (NDD) conditions including but not limited to amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD) and Parkinson’s diseases (PD) [1,2]. It occurs through oxidation of vital cellular biomarkers such as nucleic acids and proteins, crosslinking of membrane constituent and lipids of all kinds within and outside cells [3–5]. Even though a number of cell types considered H2O2 mitogenic at low concentration [6], it is oxidizable effect at overwhelming quantity often leads to the general cellular damage with consequent death via apoptosis and other processes, affecting the host organs severely [7]. This type of action is largely seen in brain cells due to their high sensitivity, high demand of energy and being the host of many peroxidizable molecules [8,9]. However, accumulation of ROS begins in the neuros prior to clinical detections of signs and symptoms of NDDs particularly AD and PD [10,11]. When that happened, apoptotic mechanism usually switches on to eliminate neurons deemed unbearable [12,13], resulting to severe morphological and functional deficit, leading to progressive decline in cognitive and memory well-being [14,15].
Interestingly, the role of reported plant sourced natural compounds with promising antioxidant and anti-inflammatory activities that prevent or delay the occurrence and progression of NDDs, has been pursuing the interest of many researchers in the quest for additional candidates with better potentials [16–18]. Having said that, Glucomoringin-isothiocyanate (GMG-ITC) was reported to have wide range of biological activities such as anti-inflammatory, anti-oxidant, antimicrobial and antiulcer [19–22]. The GMG-ITC was also reported to attenuate damages in spinal cord injury (SCI) [23], and it could be more promising candidate for neuronal protection. GMG-ITC is a hydrolytic product of a rare glucosinolate called glucomoringin (GMG) isolated from the seed of Moringa oleifera commonly known as “horse-radish tree” [20], the most popular among species under genus Moringaceae [24]. The hydrolytic reaction is catalysed by β-thioglucoside glucohydrolase (Myrosinase) (EC 3.2.1.147), a specific hydrolytic enzyme that is released as a result of damage in different parts of host plant [25]. In view of the aforementioned potentials of GMG-ITC, we therefore investigated the neuroprotective activity of GMG-ITC against H2O2-induced cytotoxicity on differentiated human neuronal cells, and assessed the surface ultrastructural and internal morphological features by means of cellular and molecular evidences, for better insight on how the compound work, which could be value added to the existing knowledge of the compound.
Materials and methods
Isolation, purification and bioactivation of glucomoringin (GMG)
GMG was isolated from the methanolic seeds extract of M. oleifera according the stipulated method reported by Rajan et al. [25]. In brief, GMG was isolated using ion exchange chromatography system and purified by gel filtration. The isolated GMG was characterised by means of proton (1H), carbon (13C) and two dimensional (2D) nuclear magnetic resonance (NMR) spectrometry. The purity of the compound was ascertain through high performance liquid chromatography (HPLC) analysis of desulfo-derivatives in line with ISO 91671 method approved by European union commission regulation, EEC No 1864/90 [26]. Molecular weight of GMG was identified using electrospray ionization (ESI) in positive mode. Additionally, 1 mg of the purely isolated GMG was dissolved in 1 ml PBS at pH 7.2 and incubated with 20 μl myrosinase enzymes (Sigma Aldrich) at 37°C. After 15 minutes of incubation, the GMG produced glucomoringin isothiocyanate (GMG-ITC) which the active compound used in the present study. However, the complete hydrolysis of GMG to GMG-ITC was confirmed by HPLC and LCMS analysis employing sinigrin as internal standard as described by Galuppo et al. [27].
Cell lines and cell cultures
SHSY5Y cells used in the present study were generously provided by UKM Medical Molecular Biology Institute (UMBI), Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia. Due to their neuron like characteristics, the cells could be fully differentiated into neuronal cells by appropriate concentration of retinoic acid (RA), thus, are suitable model for neuroprotection research. The cells were maintained in Dulbecco’s Modified Eagle Media and Hams’ 12 (DMEM/Hams’ F12) in ratio 1:1 (Nacalai, Kyoto, Japan), supplemented with 10% fetal bovine serum (FBS), 1% 2 mM essential amino acid (L-Glutamine), 1% (10000 unit/ml of penicillin and 10000 μg/ml of streptomycin) (Nacalai, Kyoto, Japan), and incubated in 5% CO2 and 95% humidified atmospheric air at 37°C.
Differentiation of SHSY5Y cells
SHSY5Y cell differentiation was performed according to the modified protocol described by Lopes et al. [28]. Briefly, the cells were seeded in 6-wells plates at a density of 1 x 105 cells/well. After 24 hours of incubation, 2mL DMEM/F12 media containing 3% heat inactivated FBS and 10 μM all trans retinoic acid (RA) was added to each well in the dark and kept in 5% CO2 incubator at 37°C. The differentiation media was changed daily for a period of seven days. At the end of the experiment, RA induced differentiation was examined under phase contrast using inverted light fluorescence microscope (Zeiss Axio Vert A1, Germany) equipped with image acquisition system (AxioCam MRm, Germany), and multiple images were captured independently. The differentiation was further confirmed by immunocytochemistry assay where expression of neuron specific class III β-tubulin was detected by means of Alexa 488 conjugated antibody.
Immunocytochemistry (ICC) assay
To further ascertain the differentiation of SHSY5Y cells into full neuronal cells by retinoic acid (RA), ICC was conducted according to the protocol enclosed in the kit as follows: the cells were seeded at 24-well plates at a density of 2 x 104 cells/well and dedifferentiated as described above. The differentiated cells were washed three times with cold phosphate buffer saline (0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride) pH 7.4, at 25 °C followed by incubation with 300 μl fixation solution (4% Paraformaldehyde “PFA”, 1M NaOH and PBS) at 25°C for 30 min and washed with PBS thereafter. Permeation solution (1% Triton X-100 and 99% PBS) and blocking (0.3% bovine serum albumin, 10% goat serum, 10% tween 20 and PBS) solution were incubated with the cells at 25°C for 15 and 30 min, accompanied with washing at each stage. Antibody for Class III β-tubulin (Tuj-1), a cytoplasmic neuron specific protein, was added in a ratio of 1:200 blocking solution with subsequent overnight incubation at 4°C. The cells were washed with PBS in the following day and incubated with Alexa fluorophore-488 secondary antibody conjugate (1:200) in the dark at 25°C for 2 hours. Then the cells were then incubated with nuclear counterstaining dye (DAPI dye) 10 min prior to image viewing under inverted light fluorescence microscope (Zeiss Axio Vert A1, Germany) equipped with image acquisition system (AxioCam MRm, Germany), where multiple images were captured independently.
Cytotoxicity and cell viability assay
GMG-ITC effect on cell viability and its ability to protect neuron cells against H2O2-induced oxidative damage coupled with the cytoxicity of H2O2 were evaluated by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay according to the modified protocol reported by Ismail et al. [29]. SHSY5Y cells were seeded at density of 1 x 104 in 96-wells plate and differentiated for seven days as described above. To assess the viability influence of GMG-ITC, the cells were incubated with serially diluted concentration (0.313–10) μg/ml of GMG-ITC for 24, 48 and 72 hours. A twenty microliter (20 μl) of MTT solution was added and the plate was incubated in the dark for 4 hours. Thereafter, the reagent was replaced with 200 μl DMSO to solubilize the formazan formed in the wells. Absorbance was measured immediately at 540 nm using microplate reader (Synergy H1, BioTek, USA). Similar analysis was conducted for H2O2 cytotoxic effect, in which 1000 μM concentration was serial diluted to 15.63 μM and the optical density was used to evaluate the IC50 of H2O2 used in the present study. Additionally, neuroprotection activity of GMG-ITC was ascertained when the differentiated SHSY5Y cells were pre-treated with serially diluted GMG-ITC (0.313–10 μg/ml) in time-dependant manner prior to four hours challenged by 300 μM (IC50) H2O2 for 4 h, followed by addition of 20 μl and 200 μl of MTT and DMSO reagents respectively. Optical density was measured at 560 nm in all respect, and the experiments were conducted in triplicates under aseptic condition.
Acridine orange and propidium iodide (AO/PI) double staining
SHSY5Y cells were seeded in 6-well plates at density of 1 x 105 cell/well and differentiated as described above. The cells were pre-treated with GMG-ITC and myrosinase separately for 72 hours and exposed to 300 μM H2O2 thereafter for 4 hours. After trypsinization, the cells were washed twice and re-suspended in PBS. A mixture of 10 μl propidium iodide (1 mg/ml) and 1 μl (10 mg/ml) acridine orange was combined with 10 μl cell suspension and transferred to glass slide after 15 min incubation at room temperature in the dark. The stained cells were examined under inverted fluorescence microscope (Zeiss Axio Vert A1, Germany) equipped with image acquisition system (AxioCam MRm, Germany). Multiple images were taken independently.
Flow cytometry analysis
Cellular death was detected using Annexin V-FITC apoptosis detection kit (BD Pharmingen, Japan) according to the protocol enclosed in the kit. Briefly, SHSY5Y cells were seeded in 6-well plate at a density of 1 x 105 cells/well, differentiated and pre-treated with GMG-ITC and myrosinase followed by 4 hours exposure to H2O2 as described above. The cells were trypsinized, washed twice with PBS, and re-suspended in 1X binding buffer (0.1 M HEPES/NaOH pH7.4, 1.4 M NaCl and 25 mM CaCl2). Mixture of 5 μl Annexin V-FITC and Propidium Iodide (PI) each was added to 40 μl cell suspension and incubated for 15 min at room temperature in the dark. A 450 μl 1X binding buffer was added to the stained cells thereafter. The content was vortex, filtered and analysed using flow cytometer (Cyan ADP, Beckman Coulter, Brea, CA, USA) equipped with Summit v4.3 software.
Scanning electron microscopy (SEM)
SEM was conducted by seeding SHSY5Y cells in T25 ml flasks at a density of 1 x 106 cells/flask and differentiated after attachment as described above. The cells were pre-treated with GMG-ITC and myrosinase separately for 72 hours and challenged with 300 H2O2 for 4 hours. Upon completion of treatment, the cells were trypsinized and washed with PBS accordingly. In house preparatory guideline for SEM obtainable at the Microscopic Unit, Institute of Bioscience, Universiti Putra Malaysia was followed vehemently. In brief, PBS washed cells were fixed with 4% glutaraldehyde and 1% osmium tetraoxide for 6 and 2 hours respectively, and the cells were washed in between with 0.1M sodium cacodylate buffer three times at the interval of 10 min each. Dehydration with 35, 50, 75 and 95% acetone was performed after discarding the fixatives. The cells were further dehydrated three times with 100% acetone and dried off on a critical dryer for 30 min. The dried pellets were coated with gold particles immediately after mounting and were viewed under scanning electron microscope (JSM 6400, Joel, USA). Multiple images were taken at different magnifications.
Transmission electron microscopy (TEM)
Likewise, TEM was carried out by seeding SHSY5Y cells in T25 ml flasks at the density of 1 x 106 cells/flask and differentiated after being attached as described above. The cells were trypsinized and washed twice with PBS after GMG-ITC and myrorinase pre-treatment coupled with H2O2 exposure. The cells were fixed with 4% glutaraldehyde and 1% osmium tetraoxide followed by dehydration using various concentration of acetone as previously mentioned. The cells were also infiltrated with a mixture of acetone and resin in a ratio of 1:1 for 60 min, 1:3 for 120 min and 100% resin overnight. Embedment was carried out by inserting the infiltrated cell in to a resin filled beam capsule. The specimen was cut in to 1 μM thick sections using an ultramicrotome after two days of polymerisation at 60 °C in the oven. Toluidine was employed to stain the sections prior to reducing the thickness of the specimen in to 60–90 nm. After uranyl acetate and lead staining for 15 and 10 min respectively, the thinner sections were viewed under transmission electron microscope (JEM-2100F, Joel, USA). Multiple images were taken at different magnifications.
Statistical analysis
Data are presented as means ± standard deviation, differences between the means of test and control groups were determined by one-way analysis of variance (ANOVA) with Tukey’s multiple compassion, on Statistical Package for Social Sciences (SPSS) software version 21 (Inc., Chicago, Illinois, USA). 95% level of confidence was considered, thus p<0.05 referred to statistical significance.
Results
Differentiation of SHSY5Y cells in to full neurons
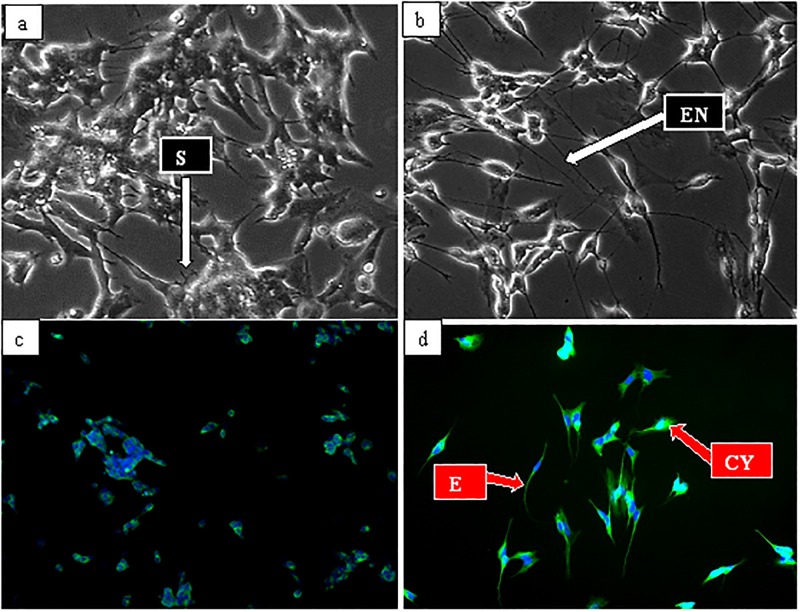
To demonstrate the transformation of SHSY5Y cells into neuronal lineage used in the present study, the 10 μM retinoic acid (RA) treated cells with extended neurites was observed after 24 hours of treatment (data not shown). The neurite features persisted and intensified after seven days of treatment (Fig 1a). Meanwhile, undifferentiated cells revealed no or comparatively smaller neurites (Fig 1b), indicating that the SHSY5Y cells were differentiated in to typical neuronal cells hence, they were used throughout the experimental analyses.
Fig 1. Micrographs of neuronal cells differentiation by 10 μM all trans retinoic acid (ATRA).
(a) Undifferentiated cells cultured in 10% complete growth media for seven (7) days and viewed under phase contrast, (b) Differentiated cells cultured in 3% heat-inactivated FBS complete growth media containing 10 μM ATRA for seven (7) days and viewed under phase contrast, and (d) expressed tuj-1 in both cytoplasm and neurites. SN = short neurites, EN = extended neurites, CYP = cytoplasm,. Magnification (x 20).
Immunocytochemical analysis of neuron specific marker’s expression
The differentiation events of SHSY5Y revealed by phase contrast microscopy was confirmed by immunocytochemistry. Where, fluorescence intensity of the expressed class III β-tubulin (tuj-1) was compared between undifferentiated and seven days RA differentiated SHSY5Y cells. The intensity of green fluorescent appeared weak and detected only in few of the undifferentiated cells (control) (Fig 1c), while that of differentiated increased markedly in both the cytoplasm and neurites (Fig 1d). The increase in green fluorescent intensity indicates high expressions of tuj-1 in the differentiated cells, thus confirming accomplishment of differentiation process.
Effect of GMG-ITC on H2O2-induced cell death in neuron cells
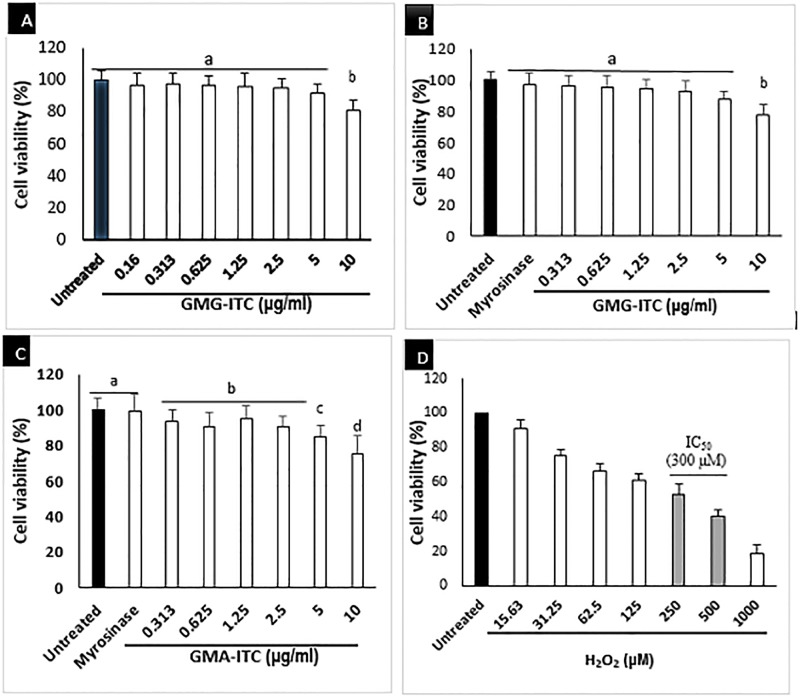
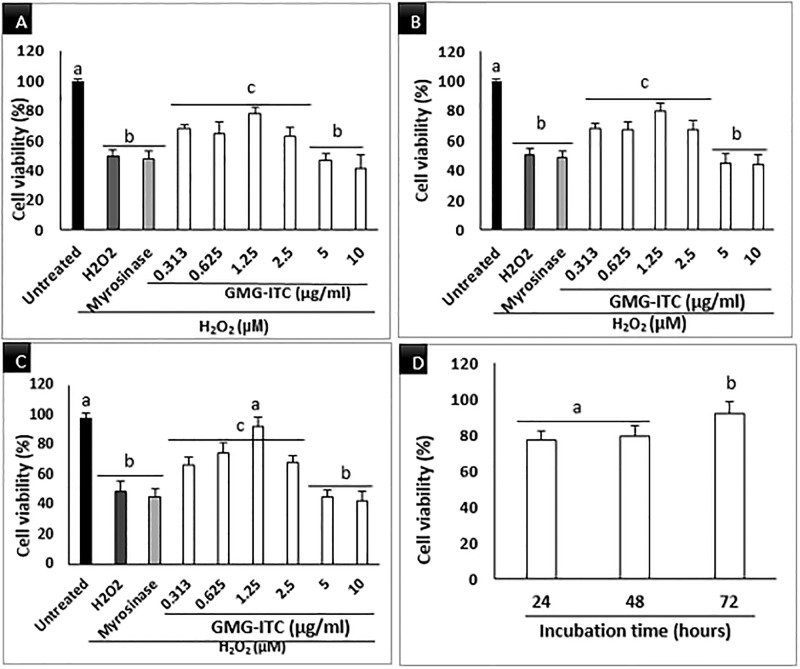
The GMG-ITC treated cells were significantly viable across the concentrations used except those treated with 10 μg/ml, where slight decrease in viability was noticed (Fig 2a, 2b & 2c). on the other hand, the differentiated cells were exposed to H2O2 at different concentrations (15.6 to 1000) μM in time dependant manner similar to [29], and the result obtained indicated that 300 μM H2O2 triggered the death of 50% of the cell population in 4 h (Fig 2d). Therefore, it was selected as the concentration of H2O2 to challenge GMG-ITC pre-treated cells in the subsequent experiments. Also, the GMG-ITC pre-treated and H2O2 exposed differentiated neuronal cells were analysed accordingly. Although the MTT analysis showed the obvious inhibition of neuronal cells’ viability by H2O2, pre-treatment of the cells with GMG-ITC provided protection to the cells against the cytotoxic effect of H2O2 across the experimental period (Fig 3a, 3b & 3c). Interestingly, pre-treatment with 1.25 μg/ml GMG-ITC demonstrated highest viability in all respect especially after 72 hours of treatment (Fig 3d). Hence, it was chosen to be used as working concentration throughout the experiments.
Fig 2. Cytotoxicity of GMG-ITC on differentiated neuronal cells at different concentrations (0.313 to 10) μg/ml.
(A) display 24 h, (B) 48 h and (C) 72 h of treatment. Whereas (D) is a cytotoxic analysis result of H2O2 used in this study with IC50 = 300 μM. Values are presented in means ± SD of triplicate experiments and means with different letters varies significantly (p<0.05).
Fig 3. Concentration dependent viability of differentiated neuronal cells, pre-treated with GMG-ITC (0.313–10 μg/mL).
(A) 24 h, (B) 48 h and (C) 72 h plus 4 h exposure to 300 μM H2O2. (D) is a means of 1.25 μg/ml GMG-ITC plus 4 h exposure to 300 μM H2O2. Values are presented in means ± SD of triplicate experiments and means with different letters varies significantly (p<0.05).
AO/PI double staining of differentiated neuron cells
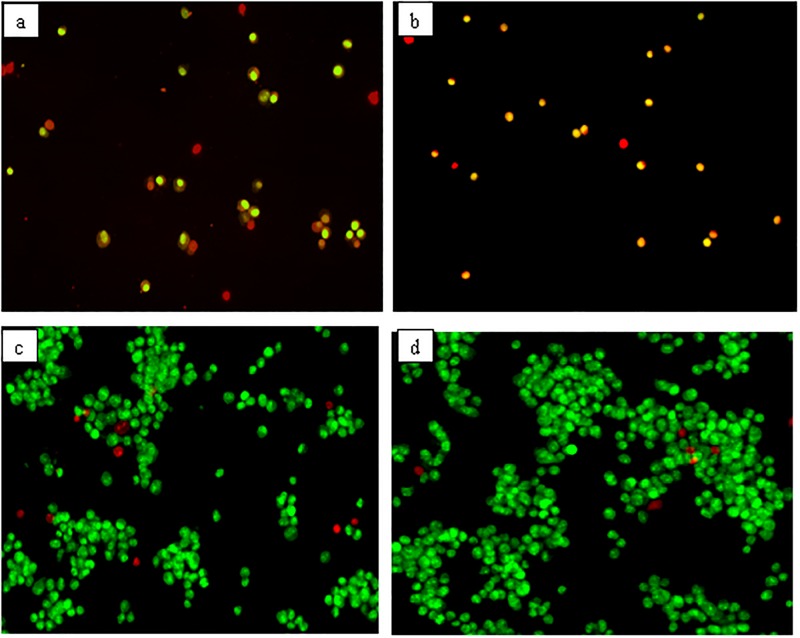
Observation of the differences between GMG-ITC pre-treated and untreated control differentiated neuron cells by means of AO and PI dyes was performed on fluorescence microscope. Green stained nucleus of the cells (Fig 4a–4d) signified viability of the cells, whereas those stained red in the same figures were unprotected against H2O2-induced cytotoxicity indicating the symbol of apoptosis. High percentage of the GMG-ITC pre-treated cells (Fig 4c) were stained green revealing normal appearance of healthy viable cells.
Fig 4. Acridine orange (AO, green) and propidium iodide (PI, red) double staining fluorescent micrographs of differentiated neuronal cells.
(a) 4 h H2O2 treated cells, (b) 72 h myrosinase pre-treated plus 4 h H2O2 exposed cells, (c) 72 h 1.25 μg/ml GMG-ITC pre-treated plus 4 h H2O2 exposed cells, (d) untreated cells (normal control). The images were captured in multiple times and x20 magnification was used.
GMG-ITC protected differentiated neurons against H2O2-induced apoptosis
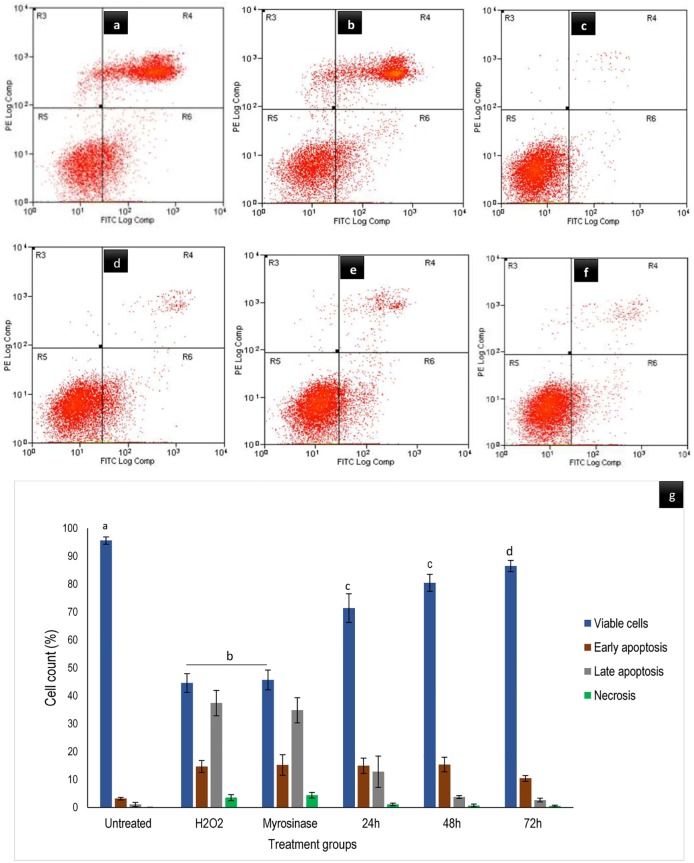
The results of flow cytometry analysis by means of annexin V-FITC and PI stains for apoptosis evaluation was obtained after GMG-ITC pre-treatment and H2O2 exposure. It was indicated that, the cells in left-lower quadrant (Annexin-V-/PI-) appeared to be healthy, those in the right-lower quadrant (Annexin-V+/PI-) seemed to underwent early apoptosis, and late apoptosis was seen in the right-upper quadrant (annexin-V+/PI+). Meanwhile necrotic process was observed in the left-upper quadrant (annexin-V-/PI+) of dot plot (Fig 5a–5f). In comparison to GMG-ITC time dependant pre-treated plus H2O2 exposure cells, percentage of apoptosis appeared to be much higher than that of necrosis in myrosinase pre-treated (enzyme control) and H2O2 alone (control) exposed cells (Fig 5g). Also, pre-treatment with 1.25 μg/ml GMG-ITC significantly lowered the early and late H2O2-induced apoptotic process with remarkable increase in the cells’ viability similar to what was observed in the untreated control cells seen in the same figure (Fig 5g).
Fig 5. Annexin V-FITC assay of differentiated neuronal cells analysed by flow cytometry.
Where (a) 4 h H2O2 treated cells, (b) 72 h myrosinase pre-treated plus 4 h H2O2 exposure cells, (c) untreated (normal control) cells, (d) GMG-ITC pre-treated for 24 h plus 4 h H2O2 exposure, (e) GMG-ITC pre-treated for 48 h plus 4 h H2O2 exposure and (f) GMG-ITC pre-treated for 72 h plus 4 h H2O2 exposure. Whereas (g) represent distribution of cells at death. Values are presented in means ± SD of triplicate experiments and means of viable cells with different letters varies significantly (p<0.05).
Surface morphological assessment of GMG-ITC pre-treated differentiated neuronal cells
Cellular surface ultrastructural analysis of differentiated neuronal cells pre-treated with or without GMG-ITC plus H2O2 exposure observed on SEM revealed an interesting outcome. Where neurites disruption, membrane blebbing and cell shrinkage were noticed on H2O2 exposed (Fig 6a) and myrosinase pre-treated plus H2O2 exposed cells (Fig 6b). However, when the cells were pre-treated with GMG-ITC prior to H2O2 exposure, their surfaces features appeared intact with folded neurites and integrated cytosol (Fig 6c). The result was similar to the untreated normal control cells seen in Fig 6d.
Fig 6. Surface morphological analysis of differentiated neuronal cells by scanning electron microscopy.
(a) 4 h H2O2 treated cells, (b) 72 h myrosinase pre-treated plus 4 h H2O2 exposure cells, (c) 72 h GMG-ITC pre-treated plus 4 h H2O2 exposure cells, (d) untreated (normal control) cells. AB = apoptotic body, IDVC = intact differentiated viable cells, FN = folded neurites, MB = membrane blabbing, NDAC = neurite disrupted apoptotic cells. Magnification (x 5000).
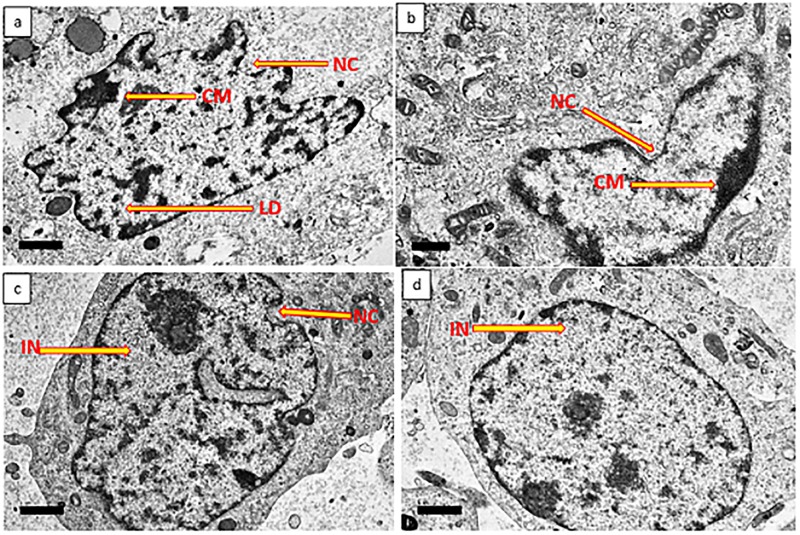
Ultrastructural analysis of GMG-ITC treated differentiated neuron cells
The ultrastructural assessment of differentiated neuronal cells performed by means of TEM showed morphological aberration in GMG-ITC untreated but H2O2 exposed cells. Where nuclear shrinkage, nuclear convolution, chromatin condensation and chromatin margination were obvious (Fig 7a). These features were absent in GMG-ITC pre-treated plus H2O2 exposure cells (Fig 7c) and untreated (normal control) cells (Fig 7d). However, the cells pre-treated with myrosinase prior to H2O2 exposure (Fig 7b) demonstrated similar features with H2O2 alone exposed cells. Again, indicating zero effect of the enzymes in neuroprotection against H2O2–induced cytotoxicity.
Fig 7. Ultrastructural analysis of differentiated neuronal cells by transmission electron microscopy.
(a) 4 h H2O2 treated cells, (b) 72 h myrosinase pre-treated plus 4 h H2O2 exposure cells, (c) 72 h GMG-ITC pre-treated plus 4 h H2O2 exposure cells, (d) untreated (normal control) cells. CM = chromatin margination, IN = intact nucleus, LD = lipid droplet, NC = nuclei convolution. Magnification (x 3000).
Discussion
The present study revealed novel insight on neuroprotection ability of an isothiocyanate of Moringa oleifera origin against H2O2-induced oxidative stress. Moringa oleifera is a medicinal plant known by many herbalists and folk medicine practitioners as “miracle tree” [30,31]. It is widely used for particularly human consumption and other domestic activities including water purification, in sub-Saharan Africa and other tropical regions worldwide [32]. Due to its richness in naturally occurring compounds, M. oleifera exhibited numerous biological and health benefits such as anti-inflammation, anticancer, antidiabetic, wound healing and antimicrobial [30,32–34]. The seeds part of the plant contains large quantity of GMG-ITC and its precursor that was reported to prevent oedema with consequent brain damage in transgenic rats [35,36]. Being a dopaminergic neuronal cell, human neuroblastoma cells (SHSY5Y) are becoming more popular as a model for neuroscience research particularly neurodegenerative diseases including but not limited to Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS) and Multiple sclerosis (MS) [37–39]. The cells develop neuronal properties such as neural extension and expression of certain neuron specific markers upon regular incubation with 10 μM all-trans retinoic acid (RA) or other differentiation inducers for appropriate period [40,41]. However, study have shown that differentiation process of SHSY5Y to full neuronal cells lower their susceptibility to cytotoxic effect of various compounds [42], thereby enhancing their stability compared to undifferentiated version of cells. H2O2-induced cytotoxicity in differentiated neuron cells resulted in cascade of reactions that overwhelmed endogenous defensive mechanism system of the cells leading to oxidative conditions with consequent cell death [43]. Although, exogenous antioxidants prevent oxidative damage by banishing ROS generation in the cells thereby increasing their chance of survival [44]. Fig 2 above showed how various concentrations of GMG-ITC enhanced viability of differentiated neurons in time dependent manner. However, 1.25 μg/ml GMG-ITC exhibited maximum potential in that respect, signifying high capability for reducing susceptibility incurred by hermetic response in the cells. The effect was obviously higher after 72 hours of treatment compared to 48 and 24 hours. This attribute to long time effect on endogenous defensive mechanism that again reduce the vulnerability of the cells to certain attacks by exogenous cytotoxic agents. Growing number of studies revealed that under normal circumstance, oxidative damage causes reactions that confer negative effect on beneficial markers in antioxidant mechanistic pathways responsible for neutralising harmful stimuli [45,46]. However, exogenous antioxidants tend to counteract such effects, but when presence in high quantity they inhibit the response generated by their indigenous counterpart thereby increasing the cells’ sensitivity to stimuli with eventual death [44].
The enhancing effect of GMG-ITC on cells’ survival was evaluated by means of AO/PI double staining, and it strengthened the earlier claim of GMG-ITC protective effect on differentiated neuron cells. Being permeable to cellular membrane, AO stains cellular nucleus green, revealing viability of the cells. Whereas the PI which is a membrane impermeable intercalating agent could only be taking up by cells with disrupted membrane, thus stained their nucleus red [12,47]. The green stained nucleus (Fig 4) indicated level of protection provided by GMG-ITC pre-treatment prior to cytotoxic induction by H2O2 that affect some of the cells (stained red or orange) observed in the same figure. Therefore, GMG-ITC demonstrated high neuroprotective activity against cellular death due to H2O2 exposure.
Furthermore, pre-treatment of GMG-ITC prevented differentiated neuronal cells against early and late apoptosis or necrosis induced by H2O2 exposure as observed in Fig 5. This indicates definite ability of the compound to keep lipid asymmetry membrane intact. Thus, preventing translocation of phosphatidylserine (PS) to cytoplasm. Although, study have shown that when cells are exposed to oxidative stress conditions, the internally generated ROS promote the disruption of membrane asymmetrical status, causing translocation of PS [48]. This effect may translate through receptor activating signals to break mitochondrial membrane potentials and trigger the release of cytochrome C with consequent cell death via apoptosis [49]. Likewise, the outcome of annexin V-FITC analyses signified that apoptosis is a predominant event occur in H2O2-induced differentiated neuron cell death. Therefore, GMG-ITC inveterate to be potential anti-apoptotic agent against H2O2-induced neuronal cell death. Also, record has it that cytotoxicity resulted in devastative cellular morphological changes such as membrane blebbing and cell shrinkage [50]. Ultrastructural surface analysis of the differentiate cells conducted by means of scanning electron microscopy (SEM) demonstrated the ability of GMG-ITC to preserve membrane integrity and protect cell surface structures including extended neurites of differentiated neurons (Fig 6c). Even though, the folded neurites on GMG-ITC pre-treated cells are highly similar to those of untreated cells, the neurites seemed to be disrupted on H2O2 exposed cells without GMG-ITC pre-treatment. On the contrary, pre-treatment with myrosinase prior to cytotoxic induction offered no effect on the differentiated cells, indicating that the observed neuroprotection against H2O2-induced oxidative damage is solely provided by GMG-ITC. This affirm our earlier claims on cell viability enhancement potential of GMG-ITC. Additionally, nuclear shrinkage, chromatin condensation and margination are typical apoptotic features in cells undergoing apoptosis [50]. As GMG-ITC prevents the occurrence of such events, we therefore postulate that the compound possessed robust neuroprotection capacity through the abolishment of internal ROS generation mechanisms.
Conclusion
In the present study, our findings highlighted the increase in viability of differentiated neuron cells in the presence of H2O2 due to GMG-ITC pre-treatment. Which perhaps facilitated through anti-apoptotic activity of the compound observed on fluorescence microscope and flow cytometry analysis. Interestingly, the result also demonstrated that GMG-ITC is capable of conserving membrane and internal structural integrity of differentiated neurons despite the exposure to oxidative damage by H2O2, indicating its strength in protecting neurons from degeneration due to oxidative stress. Therefore, this study worth expansion to obtain more evidence on how the compound provides such actions and the actual modulatory mechanistic pathways involved in the process.
Acknowledgments
The work was funded by Universiti Putra Malaysia through GP-IPS (Vot no. 9537300) and GP (Vot no. 9628600). We thank MitoMasa Sdn. Bhd., Malaysia, a leading producer and exporter of M. oleifera products for providing us with dried M. oleifera seeds sample. Our special gratitude also goes to Dr Leon Sze Wei for the vital role he played in the isolation part of this study. Also, a big thank you to Mrs Enas Mohamad Eliaser and Miss Nurul Ashikin Abd Karim for their technical assistance throughout the experimental period.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was funded by Universiti Putra Malaysia through GP-IPS (Vot no. 9537300) and GP (Vot no.9628600) to Ahmad Faizal Abdull Razis (AFAR); http://www.rmc.upm.edu.my/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weir HJ, Murray TK, Kehoe PG, Love S, Verdin EM, O’Neill MJ, et al. CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer’s disease. PLoS One. 2012. November 6;7(11):e48225 doi: 10.1371/journal.pone.0048225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolezzi JM, Silva-Alvarez C, Ordenes D, Godoy JA, Carvajal FJ, Santos MJ, et al. Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders?. PloS one. 2013. May 13;8(5):e64019 https://doi.org/10.1371/journal.pone.0064019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin L, Lenz LL, Cambier JC. Cellular reactive oxygen species inhibit MPYS induction of IFNβ. PLoS One. 2010. December 10;5(12):e15142 https://doi.org/10.1371/journal.pone.0015142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PloS one. 2013. September 4;8(9):e75044 https://doi.org/10.1371/journal.pone.0075044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PloS one. 2016. April 4;11(4):e0152925 https://doi.org/10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar AN, Bevara GB, Kaja LK, Badana AK, Malla RR. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H 2 O 2 induced cytotoxicity in lung and liver cells. BMC complementary and alternative medicine. 2016. September 29;16(1):376 doi: 10.1186/s12906-016-1354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang HC, Huang WY, Tsai TC, Hsieh WY, Ko WP, Chang KJ, et al. Supercritical fluid extract of Lycium chinense Miller root inhibition of melanin production and its potential mechanisms of action. BMC complementary and alternative medicine. 2014. June 28;14(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im AR, Chae SW, jun Zhang G, Lee MY. Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC complementary and alternative medicine. 2014. October 3;14(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Zhan L, Liang L, Sui H, Zheng L, Sun X, et al. ZiBu PiYin recipe prevents diabetes-associated cognitive decline in rats: possible involvement of ameliorating mitochondrial dysfunction, insulin resistance pathway and histopathological changes. BMC complementary and alternative medicine. 2016. July 8;16(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland D, McEvoy LK, Desikan RS, Dale AM, Alzheimer’s disease Neuroimaging Initiative. Enrichment and stratification for predementia Alzheimer disease clinical trials. PloS one. 2012. October 17;7(10):e47739 https://doi.org/10.1371/journal.pone.0047739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland D, Desikan RS, Dale AM, McEvoy LK, Alzheimer’s Disease Neuroimaging Initiative. Rates of decline in Alzheimer disease decrease with age. PloS one. 2012. August 2;7(8):e42325 https://doi.org/10.1371/journal.pone.0042325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azmi NH, Ismail N, Imam MU, Ismail M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes. BMC complementary and alternative medicine. 2013. July 17;13(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng C, Su N, Peng W, Wang Y, Chen Z, Wang Z, et al. The impact of ginsenosides on cognitive deficits in experimental animal studies of Alzheimer’s disease: a systematic review. BMC complementary and alternative medicine. 2015. December;15(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankó E, Joly O, Alzheimer’s Disease Neuroimaging Initiative. Evaluating Alzheimer’s disease progression using rate of regional hippocampal atrophy. PloS one. 2013. August 12;8(8):e71354 https://doi.org/10.1371/journal.pone.0071354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun HJ, Kwak K, Lee JM, Alzheimer’s Disease Neuroimaging Initiative. Multimodal discrimination of Alzheimer’s disease based on regional cortical atrophy and hypometabolism. PloS one. 2015. June 10;10(6):e0129250 https://doi.org/10.1371/journal.pone.0129250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacoppo S, Galuppo M, De Nicola GR, Iori R, Bramanti P, Mazzon E. Tuscan black kale sprout extract bioactivated with myrosinase: a novel natural product for neuroprotection by inflammatory and oxidative response during cerebral ischemia/reperfusion injury in rat. BMC complementary and alternative medicine. 2015. November 6;15(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Xiang L, Liu Y, Venkatraman P, Chong L, Cho J, et al. A Naturally-Derived Compound Schisandrin B Enhanced Light Sensation in the pde6c Zebrafish Model of Retinal Degeneration. PloS one. 2016. March 1;11(3):e0149663 https://doi.org/10.1371/journal.pone.0149663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman KF, Zarmouh NO, Eyunni SK. The Benzopyrone Biochanin-A as a reversible, competitive, and selective monoamine oxidase B inhibitor. BMC complementary and alternative medicine. 2017. December;17(1):34 doi: 10.1186/s12906-016-1525-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galuppo M, Giacoppo S, De Nicola GR, Iori R, Navarra M, Lombardo GE, et al. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia. 2014. June 30;95:160–74. doi: 10.1016/j.fitote.2014.03.018 [DOI] [PubMed] [Google Scholar]
- 20.Michl C, Vivarelli F, Weigl J, De Nicola GR, Canistro D, Paolini M, et al. The Chemopreventive Phytochemical Moringin Isolated from Moringa oleifera Seeds Inhibits JAK/STAT Signaling. PloS one. 2016. June 15;11(6):e0157430 https://doi.org/10.1371/journal.pone.0157430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF, et al. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PloS one. 2017. August 8;12(8):e0182658 https://doi.org/10.1371/journal.pone.0182658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PloS one. 2017. September 18;12(9):e0184709 https://doi.org/10.1371/journal.pone.0184709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giacoppo S, Galuppo M, De Nicola GR, Iori R, Bramanti P, Mazzon E. 4 (α-l-Rhamnosyloxy)-benzyl isothiocyanate, a bioactive phytochemical that attenuates secondary damage in an experimental model of spinal cord injury. Bioorganic & medicinal chemistry. 2015. January 1;23(1):80–8. [DOI] [PubMed] [Google Scholar]
- 24.Dzotam JK, Touani FK, Kuete V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC complementary and alternative medicine. 2015. December;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajan TS, De Nicola GR, Iori R, Rollin P, Bramanti P, Mazzon E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia. 2016. April 30;110:1–7. doi: 10.1016/j.fitote.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 26.Nastruzzi C, Cortesi R, Esposito E, Menegatti E, Leoni O, Iori R, et al. In vitro antiproliferative activity of isothiocyanates and nitriles generated by myrosinase-mediated hydrolysis of glucosinolates from seeds of cruciferous vegetables. Journal of agricultural and food chemistry. 2000. August 21;48(8):3572–5. [DOI] [PubMed] [Google Scholar]
- 27.Galuppo M, Nicola GR, Iori R, Dell'Utri P, Bramanti P, Mazzon E. Antibacterial activity of glucomoringin bioactivated with myrosinase against two important pathogens affecting the health of long-term patients in hospitals. Molecules. 2013. November 20;18(11):14340–8. doi: 10.3390/molecules181114340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes FM, Schröder R, da Frota Júnior ML, Zanotto-Filho A, Müller CB, Pires AS, et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain research. 2010. June 14;1337:85–94. doi: 10.1016/j.brainres.2010.03.102 [DOI] [PubMed] [Google Scholar]
- 29.Ismail N, Ismail M, Imam MU, Azmi NH, Fathy SF, Foo JB. Mechanistic basis for protection of differentiated SH-SY5Y cells by oryzanol-rich fraction against hydrogen peroxide-induced neurotoxicity. BMC complementary and alternative medicine. 2014;14:467-. doi: 10.1186/1472-6882-14-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson ME, Sankaran RP, Fahey JW, Grusak MA, Odee D, Nouman W. Leaf Protein and Mineral Concentrations across the “Miracle Tree” Genus Moringa. PloS one. 2016. July 26;11(7):e0159782 https://doi.org/10.1371/journal.pone.0159782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurber MD, Fahey JW. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the “Diffusion of Innovations” theory. Ecology of food and nutrition. 2009. May 7;48(3):212–25. doi: 10.1080/03670240902794598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdull R, Ahmad F, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pacific Journal of Cancer Prevention. 2014;15(20):8571–6. [DOI] [PubMed] [Google Scholar]
- 33.Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PloS one. 2015. August 19;10(8):e0135814 https://doi.org/10.1371/journal.pone.0135814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumssa DB, Joy EJ, Young SD, Odee DW, Ander EL, Broadley MR. Variation in the mineral element concentration of Moringa oleifera Lam. and M. stenopetala (Bak. f.) Cuf.: Role in human nutrition. PloS one. 2017. April 7;12(4):e0175503 https://doi.org/10.1371/journal.pone.0175503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galuppo M, Giacoppo S, Iori R, De Nicola GR, Bramanti P, Mazzon E. Administration of 4-(α-L-Rhamnosyloxy)-benzyl Isothiocyanate Delays Disease Phenotype in SOD1G93A Rats: A Transgenic Model of Amyotrophic Lateral Sclerosis. BioMed research international. 2015. May 5;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF, et al. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PloS one. 2017. August 8;12(8):e0182658 https://doi.org/10.1371/journal.pone.0182658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Kim SK, Kim HK, Mattson MP, Hyun DH. Mitochondrial function in human neuroblastoma cells is up-regulated and protected by NQO1, a plasma membrane redox enzyme. PloS one. 2013. July 11;8(7):e69030 https://doi.org/10.1371/journal.pone.0069030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangieri LR, Mader BJ, Thomas CE, Taylor CA, Luker AM, Tonia ET, et al. ATP6V0C knockdown in neuroblastoma cells alters autophagy-lysosome pathway function and metabolism of proteins that accumulate in neurodegenerative disease. PLoS One. 2014. April 2;9(4):e93257 https://doi.org/10.1371/journal.pone.0093257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastrantonio R, Cervelli M, Pietropaoli S, Mariottini P, Colasanti M, Persichini T. HIV-Tat induces the Nrf2/ARE pathway through NMDA receptor-elicited spermine oxidase activation in human neuroblastoma cells. PloS one. 2016. February 19;11(2):e0149802 https://doi.org/10.1371/journal.pone.0149802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, et al. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PloS one. 2013. May 28;8(5):e63862 https://doi.org/10.1371/journal.pone.0063862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson R, Subramanian V. PA6 Stromal Cell Co-Culture Enhances SH-SY5Y and VSC4. 1 Neuroblastoma Differentiation to Mature Phenotypes. PloS one. 2016. July 8;11(7):e0159051 https://doi.org/10.1371/journal.pone.0159051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009. January 31;30(1):127–35. doi: 10.1016/j.neuro.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Wang Y, Du W, Liu W, Liu F, Zhang L, et al. Wnt1 inhibits hydrogen peroxide-induced apoptosis in mouse cardiac stem cells. PloS one. 2013. March 22;8(3):e58883 https://doi.org/10.1371/journal.pone.0058883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobwala S, Fan W, Hines CJ, Folk WR, Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC complementary and alternative medicine. 2014. July;14:271-. doi: 10.1186/1472-6882-14-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans M, Paterson E, Barnes DM. An open label pilot study to evaluate the efficacy of Spanish black radish on the induction of phase I and phase II enzymes in healthy male subjects. BMC complementary and alternative medicine. 2014. December 9;14(1):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo SX, Jin YY, Fang Q, You CG, Wang XG, Hu XL, et al. Beneficial effects of hydrogen-rich saline on early burn-wound progression in rats. PLoS One. 2015. April 13;10(4):e0124897 https://doi.org/10.1371/journal.pone.0124897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu P, Li Z, Wang H, Zhang X, Yang Z. Triptolide Inhibited Cytotoxicity of Differentiated PC12 Cells Induced by Amyloid-Beta25–35 via the Autophagy Pathway. PloS one. 2015. November 10;10(11):e0142719 https://doi.org/10.1371/journal.pone.0142719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isachenko V, Todorov P, Isachenko E, Rahimi G, Tchorbanov A, Mihaylova N, et al. Long-time cooling before cryopreservation decreased translocation of phosphatidylserine (Ptd-L-Ser) in human ovarian tissue. PloS one. 2015. June 17;10(6):e0129108 https://doi.org/10.1371/journal.pone.0129108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumari A, Singh KP, Mandal A, Paswan RK, Sinha P, Das P, et al. Intracellular zinc flux causes reactive oxygen species mediated mitochondrial dysfunction leading to cell death in Leishmania donovani. PloS one. 2017. June 6;12(6):e0178800 https://doi.org/10.1371/journal.pone.0178800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waziri PM, Abdullah R, Yeap SK, Omar AR, Abdul AB, Kassim NK,et al. Clausenidin from Clausena excavata induces apoptosis in hepG2 cells via the mitochondrial pathway. Journal of ethnopharmacology. 2016. December 24;194:549–58. doi: 10.1016/j.jep.2016.10.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.