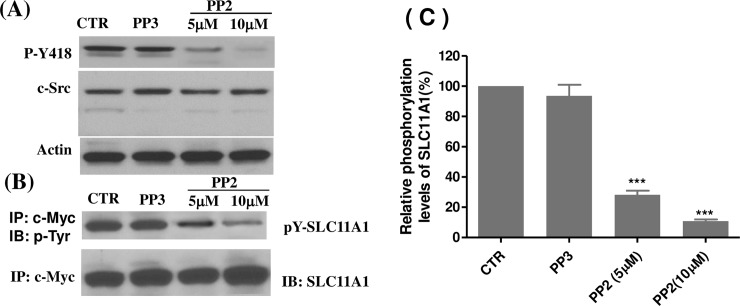
Fig 2. Inhibition of Src family kinase activity blocks tyrosine phosphorylation of SLC11A1.
U937-SLC11A1 cells were cultured with PMA (10ng/ml) for 48 hrs and were then left untreated (CTR) or treated with PP2 or PP3 (inactive analogue) for another 24 hours. Cell lysates were prepared. (A) c-Src and active c-Src (pY418) were monitored by Western blotting analysis. (B) Cell lysates were immunoprecipitated with the anti-c-Myc antibody 9E10, and the immunoprecipitates were probed with antibody 4G10 to phosphotyrosine for phosphorylated SLC11A1. The Western blots were stripped and re-probed with an antibody against SLC11A1. (C) The level of SLC11A1 phosphorylation was quantified by densitometry analysis and normalized to the level of total SLC11A1 protein. The relative phosphorylation level of SLC11A1 in untreated control group was set as 100%. The inhibitory effect of PP2 on SLC11A1 phosphorylation was assessed in 3 separate experiments (mean± SE). ***P<0.001, compared with untreated control group.