Abstract
Multiple sclerosis (MS) is a fatal demyelinating disease that primarily affects axons leading to massive neurodegeneration. Many studies have reported the causes and drawn the conclusions that multiple factors such as recurrent viral infections, hereditary link, and environmental condition are involved in the pathogenesis of MS. In essence, all these reports indicate a severe change in the biochemical milieu in the central nervous system (CNS) leading to inflammation and neurodegeneration. Recent studies in our laboratory revealed aberrant sphingolipid metabolism and accumulation of toxic sphingosine in the CNS tissues in MS patients. An elevation in sphingosine in MS brain white matter and plaque indicated that sphingosine toxicity might mediate degeneration of oligodendrocytes contributing to demyelination. An intermittent increase in ceramide followed by sphingosine accumulation in spinal cords from Lewis rats with experimental autoimmune encephalitis (EAE) and also stimulation of serine-palmitoyltransferase (SPT) activity correlated with induction of apoptosis in the lumbar spinal cord in EAE animals. Cytokine-stimulated ceramide elevation in cultured human oligodendrocytes was almost completely blocked by myriocin, an inhibitor of SPT. Myriocin exposure also protected oligodendrocytes from induction of apoptosis. Sphingosine toxicity via ceramide biosynthesis contributed to oligodendrocyte degeneration in both EAE and MS. Although many clinical trials are being conducted for MS, to the best of our knowledge, there is still no sphingolipid-targeted therapy available. Hence, we propose that sphingosine toxicity via ceramide generation may be a potential therapeutic target in both EAE and MS.
Keywords: Apoptosis, Ceramide synthesis, Central nervous system, Demyelination, Multiple sclerosis, oligodendrocytes, Serine-palmitoyltransferase, Sphingosine
Introduction
Axonal transection and axon loss are common phenomena in multiple sclerosis (MS), which is a demyelinating disease in humans. Pathological characteristics of MS include progressive demyelination and neurological deterioration[1]. Pathogenesis in MS is associated with many unknown factors such as recurrent viral infection, heredity, autoimmune demyelination, etc.[2, 3] Demyelination may proceed via different mechanisms in different MS patients[4] due to its stimulation by a variety of factors such as proteolytic enzymes, cytokines, oxidative products, and free radicals[4]. It is evident that inflammatory cytokines play a critical role in pathogenesis in MS lesion in humans and animals[5, 6] by triggering inflammation,[6, 7] though the precise mechanism of cytokine action mostly remains unresolved. Immune cell filtration accompanying chronic inflammation signifies that MS is an autoimmune demyelinating disease in the central nervous system (CNS) although the precise etiology of MS has not yet been resolved[8]. However, there remain many major unanswered issues such as primary cause of inflammation, primary target antigen, stimulation of autoimmunity, etc.[8–11] Sphingolipids including lysosphingolipids such as galactosylceramide (GalCer), ceramide, sphingoids (sphingosine or sphingenine and sphinganine), and psychosine are major components of myelin[12]. Based on studies in our laboratories, we have developed a hypothesis that sphingolipids and lysosphingolipids are metabolically altered in MS and the elevation of toxic lipids may cause apoptotic death or degeneration of oligodendrocytes and neurons.
Sphingolipids are critically involved during brain development as they stimulate cell growth, differentiation, myelinogenesis, and maintain the structural and functional integrity of the CNS and also the peripheral nervous system (PNS)[12–14]. Sphingolipid metabolism is highly regulated for normal development and function of both the CNS and PNS. Alterations in spatial and temporal levels of sphingolipids may severely compromise the maintenance of the functional integrity of the nervous system. Indeed, changes in the sphingolipid system have recently emerged as key factors in CNS disorders. An understanding of the alterations in the levels of sphingolipids and their signaling mechamism due to inflammatory responses in neurodegenerative diseases is essential in finding appropriate therapeutic agents for the treatment of these diseases. Because MS has a major inflammatory component in its pathogenesis, production and function of sphingolipids are highly affected in MS. Although the regulatory pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNFα), interferon-gamma (IFNγ), transforming growth factor-beta (TGF-β), interleukin-6 (IL-6), etc. have been implicated in fostering inflammation and demyelination in MS[8, 15, 16], their mechanism of action via sphingolipid toxicity in MS has recently been reported[17]. Both TNFα and IFNγ are highly upregulated in MS brain and EAE tissues[18, 19] and they act synergistically triggering induction of apoptosis in a wide variety of cells such as cardiomyocytes, oligodendrocytes, astroglia[20–23]. Notably, TNFα and IFNγ are elevated in MS patients after 8 weeks of endurance training[24].
Recently published reports indicated alterations in sphingolipids in MS tissues[25–27] and an earlier report suggested that sphingosine was elevated in MS brain[28]. A recent report[29] and a publication from our laboratory confirmed elevation of sphingosine in MS brain[17]. Although a correlation between MS demyelination and oligodendrocyte cell death exists[30–32] we have for the first time explored the kinetics of cytokine-mediated ceramide and sphingoid biosysthesis leading to oligodendrocyte death in MS[17].
In this review article, we discuss the cytokine-mediated sphingosine elevation triggered by de novo ceramide biosynthesis via serine-palmitoyltransferase (SPT) activation in MS[33]. In vivo ceramide is ultimately metabolized to sphingosine, indicating that de novo ceramide production may be a potential therapeutic target for treatment of MS in the future.
SPT activation and ceramide/sphingosine generation for oligodendrocyte degeneration in MS brain
We have hypothesized that aberrant sphingolipid metabolism in MS tissues may be responsible for oligodendrocyte cell death leading to demyelination. Oligodendrocytes may die in different pathological scenarios such as MS and adrenoleukodystrophy[34] as well as in other neurodegenerative diseases[35, 36]. Moreover, significant abnormalities, such as water content, astrocytic and microglial proliferation, perivascular inflammation, and occasionally, demyelination have been identified in normal appearing white matter (NAWM) in MS patients by using magnetization transfer imaging[37, 38]. Degeneration in the NAWM is a significant feature of MS and it brings about disease progression and gradual disability in MS patients. Histopathological examination of NAWM in MS patients is known to agree with the concept that areas outside the plaques harbor pathological changes that contribute to disease progression. In fact, it is now widely acknowledged that microglial activation, T cell infiltration, and perivascular cuffing occur in NAWM in MS patients. Different studies have confirmed the existence of these features in progressive MS patients. Nowadays, it is beyond reasonable doubt that a significant involvement of NAWM in progressive MS contributes to the destructive inflammation that spreads beyond central lesion areas. In a nutshell, the extent of tissue degeneration and biochemical abnormalities in white matter and the pathological features in NAWM have to be taken seriously in defining progressive MS. Axon loss typifies degeneration in NAWM. Tissue alterations and biochemical changes in NAWM precede demyelination in MS. Biochemical changes such as perturbed sphingolipid metabolism in NAWM, as reported above, is in well agreement with physico-chemical studies and may have an important implication for the disease pathogenesis. In addition, an increase in sphingosine content in NAWM and plaque in MS tissues was in compliance with previous studies that examined the MS brain sphingolipid metabolism[17, 28, 29]. However, the mechanism of sphingosine accumulation and its effect on oligodendroglia have not yet been clearly understood until our recent report[17].
Both ceramide and sphingosine accumulation in conjunction with SPT activation appeared highly prominent in the lumbar spinal cord in the experimental autoimmune encephalomyelitis (EAE) rats, the widely used animal model for MS, as we reported[17]. Hence, we have proposed that SPT activation plays a major role in cytokine mediated ceramide-sphingosine accumulation in EAE animals. New investigational drugs are always being examined to assess their effectiveness in the treatment of MS. Some new investigational therapeutics are immunomodulators such as fingolimod, laquinimod, teriflunomide, dimethyl fumarate, and cladribine that provide multiple options for treatment of MS patients. Fingolimod (or FTY720), which is an immunomodulator, as stated above, modulates ceramide metabolism and it has been introduced as a potent agent for MS therapy because its inhibitory effect on ceramide biosynthesis via ceramide synthase inhibition has clearly been demonstrated[39, 40]. Although FTY720 is a known sphingosine-1-phosphate (S1P) receptor antagonist, its role in sphingosine/ceramide regulation via S1P metabolic antagonism has not yet been examined. Since myriocin is the base structure of FTY720 (which is a myriocin derivative), we have hypothesized that FTY720 will inhibit ceramide generation (at least partly) after TNFα stimulation in a similar fashion. As we reported[17], our data appeared in agreement with our hypothesis; since FTY720 exposure of human oligodendroglioma (HOG) cells counteracted the TNFα mediated lipid alteration by reducing ceramide biosynthesis[17] and prevented cell death, as determined by fluorescent assisted cell sorting. However, the mechanism of ceramide inhibition by FTY720 differs from that of myriocin mediated SPT inhibition[40]. Myriocin blocks ceramide biosynthesis by inhibiting the SPT activation while FTY720 is more specific for inhibition of ceramide synthase (Figure 1). Specifically, FTY720 is a competitive inhibitor for ceramide synthase 2[40] and thus prevents cell death in oligodendrocytes. In fact, our studies indicate that myriocin offers more comprehensive protection than FTY720 to oligodendrocytes from cytokine-mediated apoptosis in cultured HOG cells[17].
Figure 1.
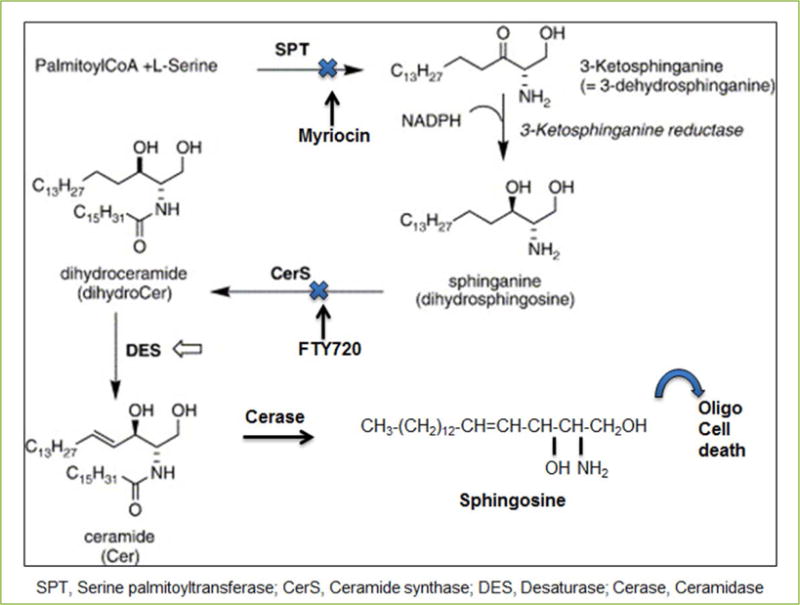
The de novo pathway for biosynthesis of ceramide and sphigosine.
Our study strongly implies that sphingosine, in addition to ceramide, is a plausible secondary mediator of signal transduction for apoptosis in oligodendrocytes leading to demyelination in MS tissues and EAE animals[17]. Although our study specifically described the effect of TNFα and IFNγ in stimulating ceramide biosynthesis (> 70%) via SPT activation leading to sphingosine accumulation[17], an alternative way of ceramide generation (salvage pathway) via sphingomyelinase activation[25] has not been ruled out. Such mechanism may also be active in sphingosine accumulation in addition to our proposed ceramide biosynthesis pathway.
It is noteworthy that our prime objective is to elucidate the ceramide/sphingosine generation in MS tissues, and we have discussed this aspect in detail with the emphasis on SPT mediated ceramide generation[17]. Clinical trials using TNFα antagonist (Lenercept) led to negative effects in MS[41]. A wide variety of inflammatory cytokines, which are released in MS, may exert an enormous devastation due to their collaborative or synergistic actions[42]. We believe that besides TNFα, stimulation of other cytokines (such as IL-1 and IFNγ) may alter metabolic property and actively degenerate oligodendrocytes and neurons triggering demyelination. Also, the strength or efficacy of the inhibitor is an important factor in preventing cell death. Based on our studies, we strongly suggest that a better approach is prevention or down regulation of the pro-inflammatory cytokine generation than an attempt to block its deleterious action, and this concept may lead to a new direction in MS research. Moreover, there are many known investigations for MS therapy using a wide variety of potentially targeted drugs (Table 1) but drugs that preferentially target to block the ceramide biosynthesis in MS have not yet been examined.
Table 1.
A list of drugs that are currently being used for MS therapy
| Drugs/Trials | Plausible target | Funding by |
|---|---|---|
| Aspirin | Permeabilization to improve fatigue | NMSS |
| Axona® | Improve cognitive function | NMSS |
| Estriol + Glatiramer acetate | Control disease course | NMSS |
| Helminth (harmless parasitic worms) | Immunomodulation to reduce disease | NMSS |
| Lipoic acid (antioxidant) | Repair optic nerve injury | NMSS |
| Phenytoin (epilepsy drug) | Prevent optic nerve injury | NMSS |
| Vitamin D + Glatiramer acetate | Reduce disease activity | NMSS |
| The MS-SMART trial | Three therapies with nerve-protecting properties in secondary-progressive MS | NMSS |
| SPRINT-MS, Ibudilast (MN-166, MediciNova, Inc.) | Anti-inflammatory agent | NMSS, Cleveland Clinic Foundation, and NeuroNEXT Network (a trial initiative of the NIH) |
| Oxcarbazepine | Neuroprotective effects in secondary-progressive MS | NMSS |
NMSS, Multiple Sclerosis Society
Ceramide biosynthesis as a potential therapeutic target for protection of oligodendrocytes in MS
We have demonstrated that sphingolipid metabolism has been altered in MS lesion as well as in NAWM. Alterations in sphingolipid metabolism in MS lesion and NAWM should be considered as an important observation. In spinal cords from EAE rats, ceramide production was transiently elevated followed by upregulation of levels of sphingosine and psychosine[17]. Furthermore, we have for the first time demonstrated an increase in ceramide and sphingosine in EAE tissues along with SPT activation and also concerted actions of TNFα and IFNγ causing sphingosine accumulation for oligodendrocyte cell death[17]. Thus, our results appeared in well agreement with previous publications that reported cytokine mediated de novo biosynthesis of ceramide[43] in other cells leading to sphingosine elevation[22, 44]. Treating the cells with myriocin (and FTY720) largely encountered the cytokine mediated aberrant sphingolipid metabolism and prevented the oligodendrocyte cell death. Hence, our findings may lead to development of novel therapeutic approaches for the treatment of devastating demyelinating diseases by targeting the ceramide biosynthetic pathway. By controlling the pro-inflammatory response and encountering the cytotoxic lipid components, MS therapy may provide highly desirable positive consequences on neuro-repair and regeneration of myelinating oligodendrocytes; however, this hypothesis has not yet been tested in MS.
Conclusions
Although no single cause has yet been established for the pathogenesis in MS, it is evident that immunological dysfunction (autoimmunity) is the most common phenomenon that leads to release of a wide variety of cytokines, some of which have deleterious effects on lipid/protein metabolism. Clinical trials using TNFα antagonist (Lenercept) showed negative effects in MS[41]. A wide variety of pro-inflammatory cytokines[45], which are released in MS tissue, may exert an enormous devastation due to their synergistic actions. Besides TNFα, stimulation of other cytokines (such as IL-1 and IFNγ) may alter lipid metabolism and thereby heavily contribute to degeneration of both oligodendrocytes and neurons. We have examined the mechanism of accumulation of sphingosine, which is a well-known toxic sphingolipid, in MS brain and the accumulation of sphingosine happens due to intermittent ceramide production via SPT activation. More research may be needed for the innovation of non-toxic drugs, which are capable of interrupting ceramide production by blocking SPT activation. Inhibition of ceramide production together with the use of immuno-suppressants may serve a sound basis for prospective MS therapy in the future. Currently available therapies and research activities are directed only to a specific site of pathogenesis while the cause of MS is far more complex. Thorough and intensive collaborative efforts involving national and international investigators may be warranted to reveal the major underlying mechanisms of pathogenesis of this devastating disease that may be targeted for long-term protection and, perhaps, an ultimate cure.
Acknowledgments
We thank late Ms. Elaine Terry and Ms. Denise Matzelle for excellent laboratory assistance. This work was supported in part by these grants: NINDS-NS-31355 (SD), NIAAA-11865 (SD), SC State Appropriation # CR22 (SD), NCI-CA-91460 (SKR), NINDS-NS-057811 (SKR), and SC SCIRF-2015-I-0 (SKR). We acknowledge Dr. Edward Hogan, Ex-chair, Department of Neurology, Medical University of South Carolina (MUSC), Charleston, SC, USA for his encouragement and also Dr. David Perry, Department of Biochemistry, MUSC, Charleston, SC, USA for his insights into the SPT assay.
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
Author contributions
SD planned, supervised, and conducted the majority of the planned experiments. SKR was involved in supervising the EAE model and conducting the experiments using the EAE tissues. Both authors equally contributed to writing and revising the manuscript.
References
- 1.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–31. doi: 10.1212/01.wnl.0000275229.13012.32. discussion S43–54. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: Implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 3.Fox RJ, Bethoux F, Goldman MD, Cohen JA. Multiple sclerosis: advances in understanding, diagnosing, and treating the underlying disease. Cleve Clin J Med. 2006;73:91–102. doi: 10.3949/ccjm.73.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Söderström M. Clues to the immunopathogenesis of multiple sclerosis by investigating untreated patients during the very early stage of disease. Neurol Sci. 2001;22:145–149. doi: 10.1007/s100720170013. [DOI] [PubMed] [Google Scholar]
- 6.Martino G, Furlan R, Brambilla E, Bergami A, Ruffini F, Gironi M, et al. Cytokines and immunity in multiple sclerosis: The dual signal hypothesis. J Neuroimmunol. 2000;109:3–9. doi: 10.1016/s0165-5728(00)00295-2. [DOI] [PubMed] [Google Scholar]
- 7.Martino G, Poliani PL, Furlan R, Marconi P, Glorioso JC, Adorini L, et al. Cytokine therapy in immune-mediated demyelinating diseases of the central nervous system: A novel gene therapy approach. J Neuroimmunol. 2000;107:184–190. doi: 10.1016/s0165-5728(00)00236-8. [DOI] [PubMed] [Google Scholar]
- 8.Deckx N, Lee WP, Berneman ZN, Cools N. Neuroendocrine immunoregulation in multiple sclerosis. Clin Dev Immunol. 2013;2013:705232. doi: 10.1155/2013/705232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff RM. Mechanisms of inflammation in MS tissue: Adhesion molecules and chemokines. J Neuroimmunol. 1999;98:57–68. doi: 10.1016/s0165-5728(99)00082-x. [DOI] [PubMed] [Google Scholar]
- 11.Kieseier BC, Storch MK, Archelos JJ, Martino G, Hartung HP. Effector pathways in immune mediated central nervous system demyelination. Curr Opin Neurol. 1999;12:323–336. doi: 10.1097/00019052-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta S, Hogan EL. Chromatographic resolution and quantitative assay of CNS tissue sphingoids and sphingolipids. J Lipid Res. 2001;42:301–308. [PubMed] [Google Scholar]
- 13.Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 15.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jana A, Pahan K. Sphingolipids in multiple sclerosis. Neuromolecular Med. 2010;12:351–361. doi: 10.1007/s12017-010-8128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller LG, Young JA, Ray SK, Wang G, Purohit S, Banik NL, et al. Sphingosine toxicity in EAE and MS: Evidence for ceramide generation via serine-palmitoyltransferase activation. Neurochem Res. 2017;42:2755–2768. doi: 10.1007/s11064-017-2280-2. [DOI] [PubMed] [Google Scholar]
- 18.Kunz M, Ibrahim SM. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009;2009:979258. doi: 10.1155/2009/979258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng LF, Ghirnikar RS, Lee YL. Inflammation in EAE: Role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res. 1996;21:511–525. doi: 10.1007/BF02527717. [DOI] [PubMed] [Google Scholar]
- 20.Bhat NR, Zhang P. Activation of mitogen-activated protein kinases in oligodendrocytes. J Neurochem. 1996;66:1986–1994. doi: 10.1046/j.1471-4159.1996.66051986.x. [DOI] [PubMed] [Google Scholar]
- 21.Andrews T, Zhang P, Bhat NR. TNF-alpha potentiates IFN-gamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández M, Bayón Y, Crespo MS, Nieto ML. Signaling mechanisms involved in the activation of arachidonic acid metabolism in human astrocytoma cells by tumor necrosis factor-α. J Neurochem. 1999;73:1641–1649. doi: 10.1046/j.1471-4159.1999.0731641.x. [DOI] [PubMed] [Google Scholar]
- 24.Castellano V, Patel DI, White LJ. Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol (1985) 2008;104:1697–1702. doi: 10.1152/japplphysiol.00954.2007. [DOI] [PubMed] [Google Scholar]
- 25.Haughey NJ. Sphingolipids in neurodegeneration. Neuromolecular Med. 2010;12:301–305. doi: 10.1007/s12017-010-8135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter S, Fassbender K. Spingolipids in multiple sclerosis. Cell Physiol Biochem. 2010;26:49–56. doi: 10.1159/000315105. [DOI] [PubMed] [Google Scholar]
- 28.Moscatelli EA, Isaacson E. Gas liquid chromatographic analysis of sphingosine bases in sphingolipids of human normal and multiple sclerosis cerebral white matter. Lipids. 1969;4:550–555. doi: 10.1007/BF02531040. [DOI] [PubMed] [Google Scholar]
- 29.Qin J, Berdyshev E, Goya J, Natarajan V, Dawson G. Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: Significance for apoptosis and multiple sclerosis. J Biol Chem. 2010;285:14134–14143. doi: 10.1074/jbc.M109.076810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 31.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: Distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 32.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: Characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 34.Clemente D, Ortega MC, Melero-Jerez C, de Castro F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Front Cell Neurosci. 2013;7:268. doi: 10.3389/fncel.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338:491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology. 1995;45:478–482. doi: 10.1212/wnl.45.3.478. [DOI] [PubMed] [Google Scholar]
- 38.Filippi M. Linking structural, metabolic and functional changes in multiple sclerosis. Eur J Neurol. 2001;8:291–297. doi: 10.1046/j.1468-1331.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog fty720 via a mixture of uncompetitive and noncompetitive inhibition in an acyl-coa chain length-dependent manner. J Biol Chem. 2009;284:16090–16098. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, et al. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Aiuto F, Parkar M, Brett PM, Ready D, Tonetti MS. Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine. 2004;28:29–34. doi: 10.1016/j.cyto.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Wiendl H, Neuhaus O, Kappos L, Hohlfeld R. Multiple sclerosis. Current review of failed and discontinued clinical trials of drug treatment. Nervenarzt. 2000;71:597–610. doi: 10.1007/s001150050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffmann S, Ferreiros N, Birod K, Eberle M, Schreiber Y, Pfeilschifter W, et al. Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2012;188:5723–5733. doi: 10.4049/jimmunol.1103109. [DOI] [PubMed] [Google Scholar]
- 44.Ullio C, Casas J, Brunk U, Sala G, Fabrias G, Ghidoni R, et al. Sphingosine mediates tnfalpha-induced lysosomal membrane cell death in hepatoma cells. J Lipid Res. 2012;53:1134–1143. doi: 10.1194/jlr.M022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiendl H, Neuhaus O, Kappos L, Hohlfeld R. Multiple sclerosis. Current review of failed and discontinued clinical trials of drug treatment. Nervenarzt. 2000;71:597–610. doi: 10.1007/s001150050636. [DOI] [PMC free article] [PubMed] [Google Scholar]


