Abstract
A previous study in humans demonstrated the sustained inhibitory effects of donepezil on acetylcholinesterase (AChE) activity; however, the effective concentration of donepezil in humans and animals is unclear. This study aimed to characterize the effective concentration of donepezil on AChE inhibition and impaired learning and memory in rodents. A pharmacokinetic study of donepezil showed a mean peak plasma concentration of donepezil after oral treatment (3 and 10 mg/kg) of approximately 1.2 ± 0.4 h and 1.4 ± 0.5 h, respectively; absolute bioavailability was calculated as 3.6%. Further, AChE activity was inhibited by increasing plasma concentrations of donepezil, and a maximum inhibition of 31.5 ± 5.7% was observed after donepezil treatment in hairless rats. Plasma AChE activity was negatively correlated with plasma donepezil concentration. The pharmacological effects of donepezil are dependent upon its concentration and AChE activity; therefore, we assessed the effects of donepezil on learning and memory using a Y-maze in mice. Donepezil treatment (3 mg/kg) significantly prevented the progression of scopolamine-induced memory impairment in mice. As the concentration of donepezil in the brain increased, the recovery of spontaneous alternations also improved; maximal improvement was observed at 46.5 ± 3.5 ng/g in the brain. In conclusion, our findings suggest that the AChE inhibitory activity and pharmacological effects of donepezil can be predicted by the concentration of donepezil. Further, 46.5 ± 3.5 ng/g donepezil is an efficacious target concentration in the brain for treating learning and memory impairment in rodents.
Keywords: Acetylcholinesterase activity, Donepezil, Pharmacokinetic, Pharmacodynamic, Y-maze
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurological disorder and is the most common cause of senile dementia, as characterized by loss of memory and other intellectual abilities that interfere with daily life (Small et al., 1997). It has been estimated that 35.6 million people lived with dementia worldwide in 2010; these numbers are expected to nearly double every 20 years to 65.7 million in 2030 and 115.4 million in 2050 (Prince et al., 2013). Although little is known regarding the cause of AD, it is generally accepted that many of its symptoms are related to a cholinergic deficit, and the extent of neuropathological features have been found to correlate with cholinergic loss in the central nervous system (Davis et al., 1995; Rogers et al., 1998).
As acetylcholinesterase (AChE) is found predominantly in the brain, striated muscle, and blood, AD treatment strategies are mainly directed toward AChE inhibition (Sozio et al., 2012).
Pharmacological effects result from the integration of pharmacokinetics (PK) and pharmacodynamics (PD) (Ito et al., 2010). The relationship between PK and PD is usually addressed by measuring plasma drug concentrations and PD markers. AChE inhibition in blood has been used as a peripheral marker for the activity of centrally acting AChE inhibitors in the treatment of AD (Sramek and Cutler, 2000). Further, AChE inhibition in blood reflects the central PD activity of the compound and demonstrates a relationship with cognitive or global improvement in AD (Rogers et al., 1998).
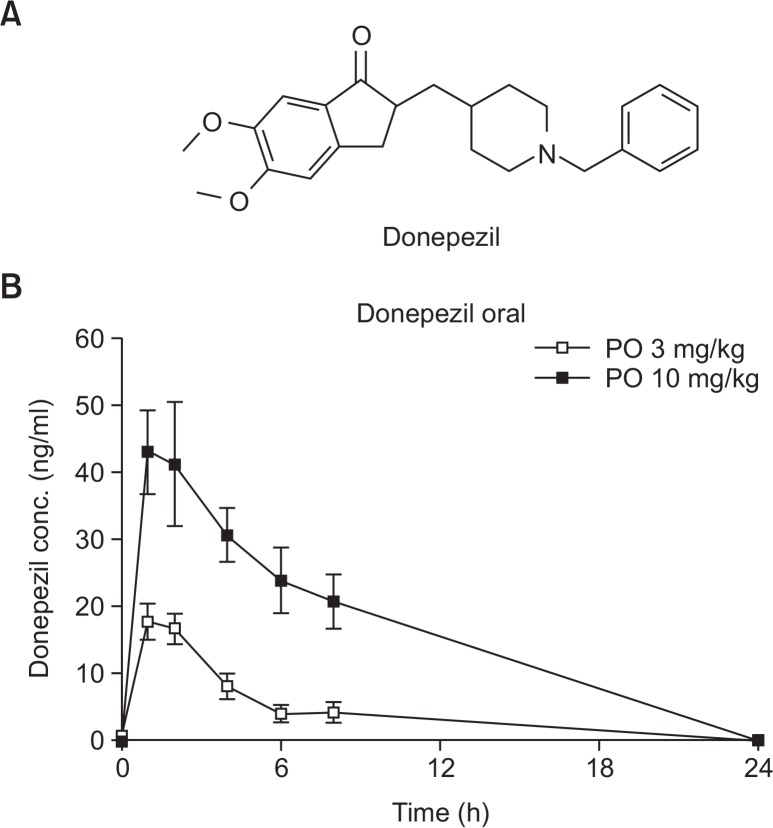
Donepezil (C24H29NO3, MW: 379.492) is a reversible, selective AChE inhibitor that is currently approved for the symptomatic treatment of AD (Fig. 1A); it is believed to inhibit the breakdown of the neurotransmitter acetylcholine (ACh) and compensate for the deficiency of ACh in the brain (Colovic et al., 2013). Donepezil was proven to improve cognitive function in mild to severely moderate AD patients in clinical trials and showed excellent tolerability without hepatotoxic effects (Rogers et al., 1998).
Fig. 1.
Structure of donepezil (A) and plasma donepezil concentrations (mean ± SD) following an oral (3 or 10 mg/kg) treatment in hairless rats.
Although previous studies in humans (Rogers et al., 1998) and rats (Goh et al., 2011) demonstrated sustained inhibitory effects of donepezil on AChE, the effective concentration of donepezil resulting in improved efficacy in the brain, as well as its relation to changes in the concentration of plasma donepezil are still unclear.
In this study, we analyzed the PK and PD of donepezil to predict the effective concentration of donepezil for AChE inhibition. Furthermore, we evaluated donepezil-associated improvements to spatial memory deficits caused by scopolamine in mice to determine the brain concentration at which significant improvements in behavioral pharmacology can be observed.
MATERIALS AND METHODS
Materials
Donepezil HCl was obtained from Aurobindo Pharma (Telangana, India). All other chemicals were purchased from Sigma-Aldrich unless otherwise noted (St. Louis, MO, USA).
Ethics statement
All animal studies were approved by the Institutional Animal Care and Use Committee of Dong-A ST Research Institute (IACCU approval No. I-1506197, I-1701030). Male hairless rats and male imprinting control region (ICR) mice (6 weeks old) were obtained from Central Lab Animal Inc. (Seoul, Korea) and Dae Han Biolink (Eumseong, Korea), respectively. Animals were acclimated for one week, maintained at 23 ± 2°C on a 12:12-h light-dark cycle (lights on 07:00–19:00), and allowed free access to food and water.
Pharmacokinetic study of donepezil in hairless rats
Hairless rats, with an average weight of 300 g, were randomly assigned to a treatment group (4–7 animals per group). Donepezil HCl (10 mg/kg free drug dissolved in saline) was administered orally, and blood (250 μl) was collected through the tail vein. Sampling continued at the indicated time points until 24 h; samples were maintained at −80°C until liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. To assess absolute bioavailability, donepezil HCl (3 mg/kg free drug dissolved in saline) was administered intravenously via the tail vein.
AChE activity assay in rat plasma
Blood samples for the AChE assay were collected from intact hairless rats and placed into heparin-filled tubes. Blood was centrifuged (1,000 × g, 4°C, 10 min) to obtain plasma, and the samples were then prepared by adding 900 μl assay buffer to 100 μl plasma. Samples were aliquoted into tubes (80 μl), and different concentrations of donepezil (from 0 to 1500 ng/ml, with a final volume of 20 μl) were incubated with the plasma samples. AChE activity was measured using a colorimetric enzyme-linked immunoassay (ELISA) kit from Abcam (Cambridge, MA, USA).
PK and PD study in hairless rats
To assess the correlation between PK and PD, hairless rats with an average weight of 300–320 g were randomly assigned to a treatment group (6 rats per group). Donepezil (5 mg free drug per rat [approximately equal to 17.9 mg/kg]) was administered orally. Blood was collected from the tail vein at each indicated time point (0, 0.5, 1, 2, 4, 6, 8, and 24 h) in a heparinfilled tube; the plasma was then separated by centrifugation. Plasma was maintained at −80°C until donepezil concentrations or AChE activity was analyzed.
PK and PD study in mice
To assess the correlation between PK and PD, ICR mice (average weight of 30 g) were randomly assigned to a treatment group (6–8 animals per group). Donepezil (approximately 0.1–10 mg/kg free drug) was administered orally. The mice were anesthetized after 1 h, and blood was collected from the orbital venous sinus in a heparin-filled tube; whole brain samples were then immediately extracted and briefly rinsed with water, dissected into halves, and stored in tubes (Geerts et al., 2005; Goh et al., 2011). Mouse brains were transferred to 2-ml capped, round-bottomed Eppendorf (E)-tubes that were pre-labeled. Purified water (4 fold the brain weight) was added to each tube, which was subsequently vortexed for approximately 15 s and then sonicated for 3 min. Methanol (1 fold the brain weight) was then added; the tube was again vortexed for approximately 5 s and then rotary mixed for 30 min. All tubes were kept at −80°C until donepezil concentrations were analyzed.
Analysis of plasma and brain donepezil levels
Analysis of plasma and brain donepezil concentrations was performed using LC-MS/MS, with a slight modification from a previously reported method (Geerts et al., 2005; Bhateria et al., 2015). Briefly, donepezil HCl (10 mg free drug) was accurately weighed into a 20-ml volumetric vial and dissolved in methanol to give a working stock solution of 1,000 μg/ml. An aliquot (100 μl) of working stock solution (1,000 μg/ml) was transferred to a 1-ml E-tube and serially diluted with methanol to give working solutions from 500 ng/ml to 3.9 ng/ml. Depending on the calibration range and matrix used (plasma or brain homogenate), samples were prepared in the following manner. A suitable aliquot of sample or control matrix was accurately pipetted into a 2-ml tube and spiked with a combination internal standard solution (amantadine 250 ng/ml; 300 μl). For calibration and quality control (QC) samples, a suitable volume of calibration and QC-spiking solution was also added. Standards were diluted to a final concentration ranging from 50 ng/ml to 0.39 ng/ml. All samples were analyzed by LC-MS/MS using a high-performance liquid chromatography (HPLC) system comprising the Agilent Technologies 1200 series coupled to a 6430 Triple Quad LC/MS (Santa Clara, CA, USA). Chromatography was carried out on a Union UK-C18 3 μ column (50×2.0 mm; Portland, OR, USA). The sample aliquot injected onto the LC-MS/MS system was 5 μl, with an auto sampler needle wash using methanol. A gradient HPLC system was used with mobile phases of (A) aqueous, formic acid (0.5%) and (B) methanol; the gradient included a total flow rate of 0.2 ml/min. Data were obtained using proprietary software from the instrument manufacturer; peak area and quantitative data were generated by MassHunter Workstation Software Quantitative Analysis, version B 04.00 (Agilent Technologies, Santa Clara).
Scopolamine-induced learning and memory impairments in mice (Y-maze)
The Y-maze task was performed according to a slightly modified method described by (Moon et al., 2013). Donepezil HCl (0.3–10 mg/kg free drug dissolved in saline) was administered orally for four consecutive days to the corresponding treatment groups (14–16 animals per group). Scopolamine (1.0 mg/kg, subcutaneous [s.c.]) was administered 30 min after the fourth dose of donepezil HCl. After 30 minutes of scopolamine administration, the mice were placed in a Y-maze and observed. The Y-maze consisted of three identical acrylic arms (30 cm long ×8 cm wide ×15 cm high) resulting in pathways separated by 120° and extending from a central space (8×8 cm). Arm entry was monitored by placing a mouse in the center of the Y-maze and allowing it to move freely for 5 min. An arm entry was counted when all 4 paws of the mouse completely entered an arm. The sequence of arm entry was calculated as alternating behavior and % alternation. Alternation was defined as a sequential visit to three different arms. Percent spontaneous alternation (SA) as a measurement of shortterm spatial memory was calculated by the following equation: SA (%)=successive entries/(total arm entries −2)×100. Percent recovery of SAs was calculated by the following equation: recovery of SA (%)=(% SA of donepezil−% SA of control]/(% SA of normal−% SA of control)×100.
Statistical analyses
All data are expressed as means ± SD. All statistical analyses were performed using SigmaStat® 2.0 (SPSS, Chicago, IL, USA). Comparisons between two groups were analyzed using student’s t-test. For comparisons of data from more than three groups at the same time point, one-way analysis of variance (ANOVA) was used. When ANOVA indicated a significant difference, the differences were further evaluated using the student-Newman-Keuls multiple comparison test. Correlations between two parameters were analyzed with Pearson’s correlation. A p-value<0.05 was considered significant.
RESULTS
PK profiles of donepezil dosed orally or intravenously
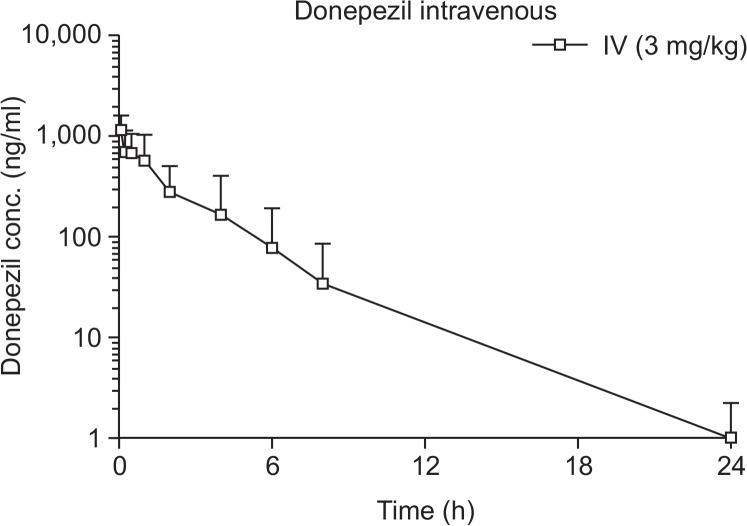
The concentration of donepezil in rat plasma was measured from 0 to 24 h after oral or intravenous (IV) administration of donepezil HCl. Plasma donepezil concentrations following oral administration are shown in Fig. 1B, with calculated PK profiles listed in Table 1. After oral treatment (3 and 10 mg/kg), a maximum concentration (Cmax) was reached after approximately 1.2 ± 0.4 h and 1.4 ± 0.5 h, respectively, and gradually decreased (Fig. 1B). The Cmax and area under the curve (AUC) were 17.9 ± 2.4 ng/ml and 70.7 ± 11.2 ng×h/ml at 3 mg/kg, and 44.1 ± 7.9 ng/ml and 240.5 ± 31.5 ng×h/ml at 10 mg/kg, respectively. To calculate the absolute bioavailability of donepezil, an intravenous bolus treatment of 3 mg/ kg was administered to hairless rats via the tail vein (Fig. 2). The Cmax and AUC were 1147.3 ± 233.4 ng/ml and 1995.3 ± 1735.3 ng×h/ml, respectively, at 3 mg/kg (Table 2). Absolute bioavailability was calculated according to a slightly modified method described by Saluja et al. (2013), and was determined to be 3.6%, indicating that the absolute bioavailability of donepezil is low.
Table 1.
Donepezil pharmacokinetic parameters after oral treatment to hairless rats (mean ± SD)
| Parameter | PO (3 mg/kg) | PO (10 mg/kg) |
|---|---|---|
| Tmax (h) | 1.2 ± 0.4 | 1.4 ± 0.5 |
| Cmax (ng/ml) | 17.9 ± 2.4 | 44.1 ± 7.9 |
| T1/2 (h) | 8.5 ± 1.9 | 3.4 ± 1.0 |
| AUC0→inf (ng×h/ml) | 70.9 ± 11.2 | 240.5 ± 31.5 |
| BA (%) | 3.6 | |
Absolute bioavailability (BA) was calculated according to a slightly modified method described Saluja et al (2013).
Fig. 2.
Plasma donepezil concentrations (mean ± SD) following an intravenous bolus (3 mg/kg) treatment in hairless rats.
Table 2.
Donepezil pharmacokinetic parameters after intravenous injection to hairless rats (mean ± SD)
| Parameter | IV (3 mg/kg) |
|---|---|
| Tmax (h) | - |
| Cmax (ng/ml) | 1147.3 ± 233.4 |
| AUC0→inf (ng×h/ml) | 1995.3 ± 1735.3 |
| Clearance (ml/h/kg) | 2501 ± 1518 |
| Volume distribution (l/kg) | 5.1 ± 3.2 |
Relationship between plasma concentration of donepezil and its inhibitory effect on plasma AChE activity
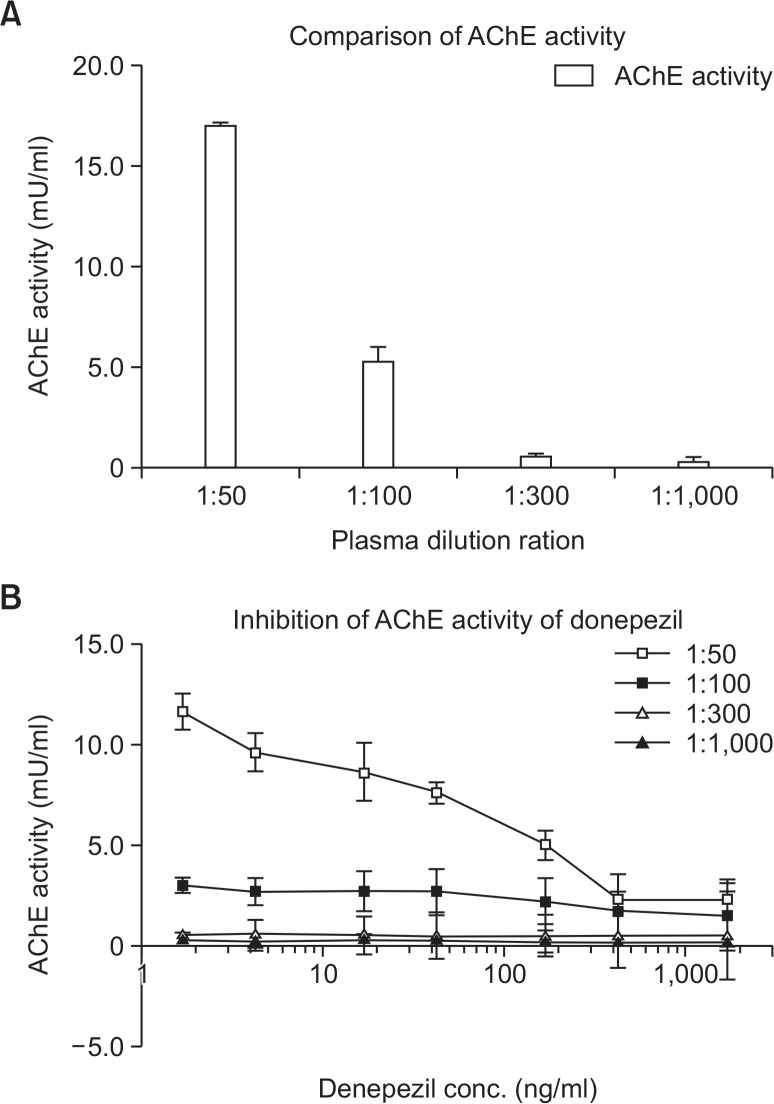
We analyzed the correlation between plasma donepezil and AChE activity ex vivo. AChE activity was measured as a plasma dilution ratio to find optimal plasma dilution conditions. When plasma was diluted 1:50, AChE activity was measured as 17.0 ± 0.1 mU/ml (Fig. 3A). Donepezil-mediated inhibition of AChE activity was observed to be concentration dependent at a dilution ratio of 1:50 (Fig. 3B).
Fig. 3.
Optimization of the plasma dilution ratio for measuring acetylcholinesterase (AChE) activity. AChE activity was measured as a plasma dilution ratio to find the optimal plasma dilution conditions. When plasma was diluted 1:50, AChE activity was measured as 17.0 ± 0.1 mU/ml (A). Inhibition of AChE activity was also observed in a donepezil concentration-dependent manner at a dilution ratio of 1:50 (B). Data represent the means ± SEM.
Once a plasma dilution ratio of 1:50 was selected, AChE activity was measured after treatment with donepezil (final concentration of 0–300 ng/ml) in intact rat plasma to predict the correlation between PK and PD in vivo. AChE activity was measured 1 h after drug treatment. As donepezil concentrations increased, AChE activity decreased; a 40% maximal inhibition was observed with 30–100 ng/ml donepezil (Fig. 4).
Fig. 4.
Ex vivo inhibitory effects of donepezil on plasma cholinesterase activity. Acetylcholinesterase (AChE) activity was measured 1 h after spiking each plasma sample with drug. Data represent the means ± SEM.
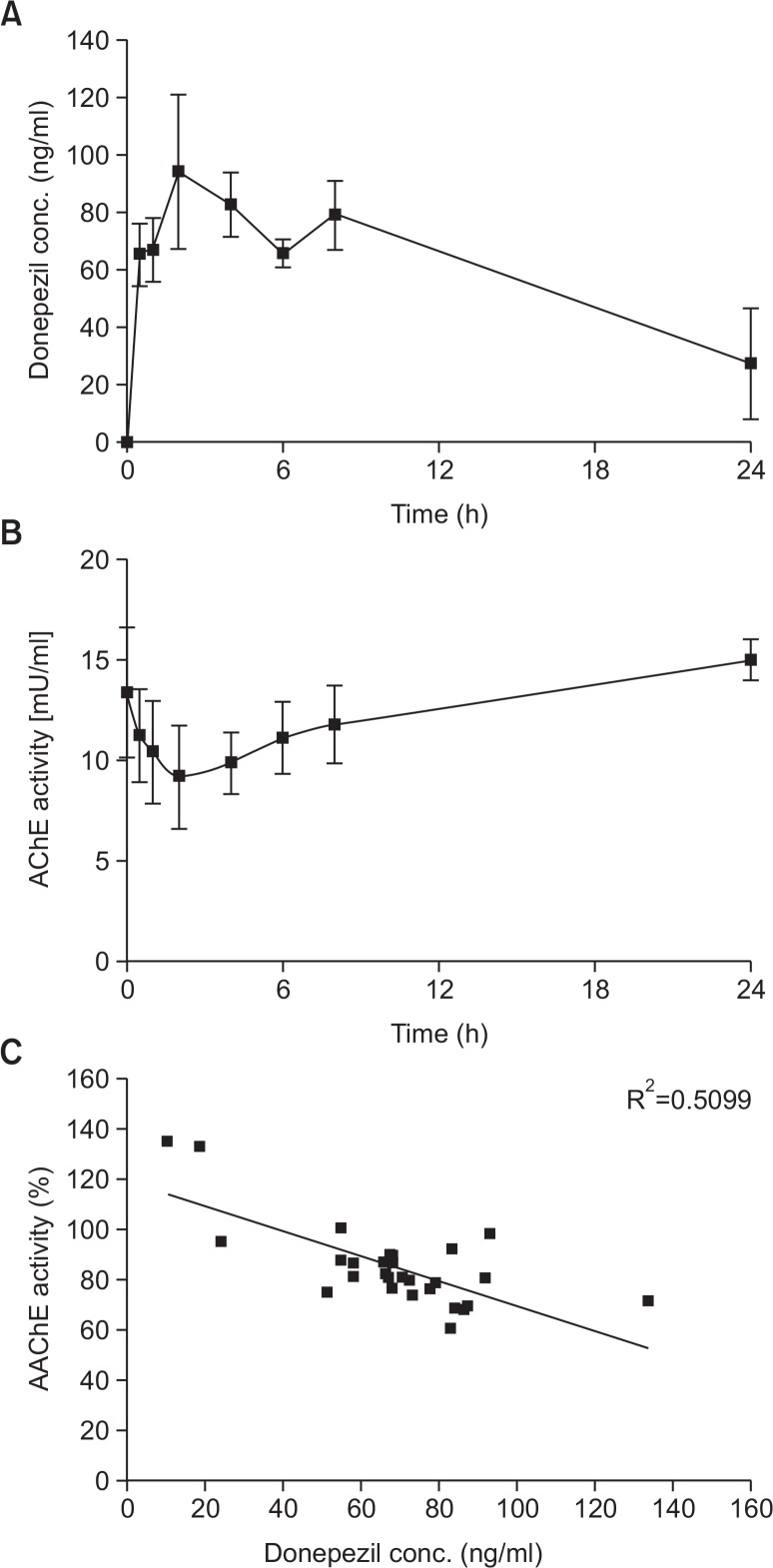
Next, we evaluated PK/PD in hairless rats. After oral treatment, the Cmax of donepezil was reached after approximately 2.5 ± 0.5 h and gradually decreased (Fig. 5A). The Cmax and AUC were 97.3 ± 24.4 ng/ml and 1342.6 ± 115.5 ng*h/ml, respectively (Table 3). AChE activity in rat plasma was also measured from 0 to 24 h after oral treatment of donepezil (Fig. 5B). AChE activity was inhibited in the presence of increasing plasma concentrations of donepezil; maximal inhibition (31.5 ± 5.7%) was observed 2 h after donepezil treatment. Plasma AChE activity levels were negatively correlated with the concentration of plasma donepezil (Pearson’s correlation coefficient R2=0.5099, p<0.001) (Fig. 5C). These results suggest that the concentration of plasma donepezil is a predictive marker for the pharmacological effects of AChE.
Fig. 5.
Correlation between cholinesterase inhibition and plasma concentration of donepezil. After oral treatment, the maximal drug concentration (Cmax) was reached after approximately 2.5 ± 0.5 h and gradually decreased (A). AChE activity was inhibited by increasing plasma concentrations of donepezil (B). Plasma AChE activity levels were negatively correlated with the concentration of donepezil in the plasma (C).
Table 3.
Donepezil pharmacokinetic parameters after oral treatment to hairless rats (mean ± SD)
| Parameter | PO (5 mg/head ≅ 17.9 mg/kg) |
|---|---|
| Tmax (h) | 2.5 ± 0.5 |
| Cmax (ng/ml) | 97.3 ± 24.4 |
| AUC0→inf (ng×h/ml) | 1342.6 ± 115.5 |
Effects of donepezil on scopolamine-induced learning and memory impairment in mice
To assess the effects of donepezil on learning and memory, we conducted Y-maze tests in mice. Mice treated with scopolamine (1.0 mg/kg, intraperitoneal [i.p.]) showed fewer SAs than the control mice (Fig. 6). However, pretreatment with donepezil (3–10 mg/kg) ameliorated scopolamine-induced memory impairment. These results suggest that donepezil treatment at doses of at least 3 mg/kg prevent the progression of memory impairment induced by scopolamine in mice.
Fig. 6.
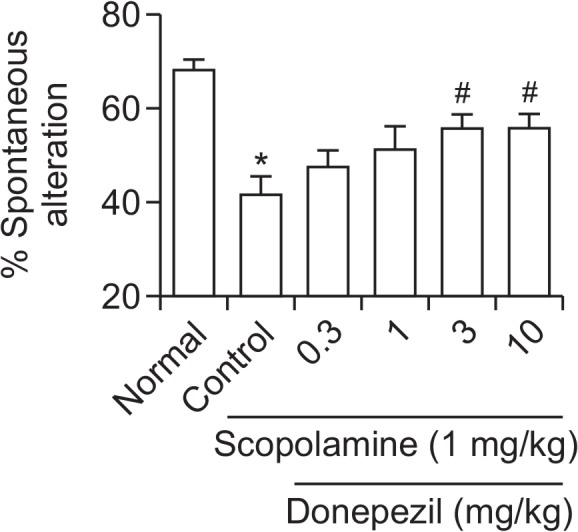
Effects of donepezil on scopolamine-induced learning and memory impairment in mice treated with scopolamine (1.0 mg/kg, i.p.). There were significantly fewer spontaneous alternations in scopolamine treated mice than in untreated control mice. However, pretreatment with donepezil (3–10 mg/kg) ameliorated the scopolamine-induced memory impairment. *p<0.05 vs. Normal, #p<0.05 vs. Control.
Relationship between brain concentration of donepezil and its pharmacological effects
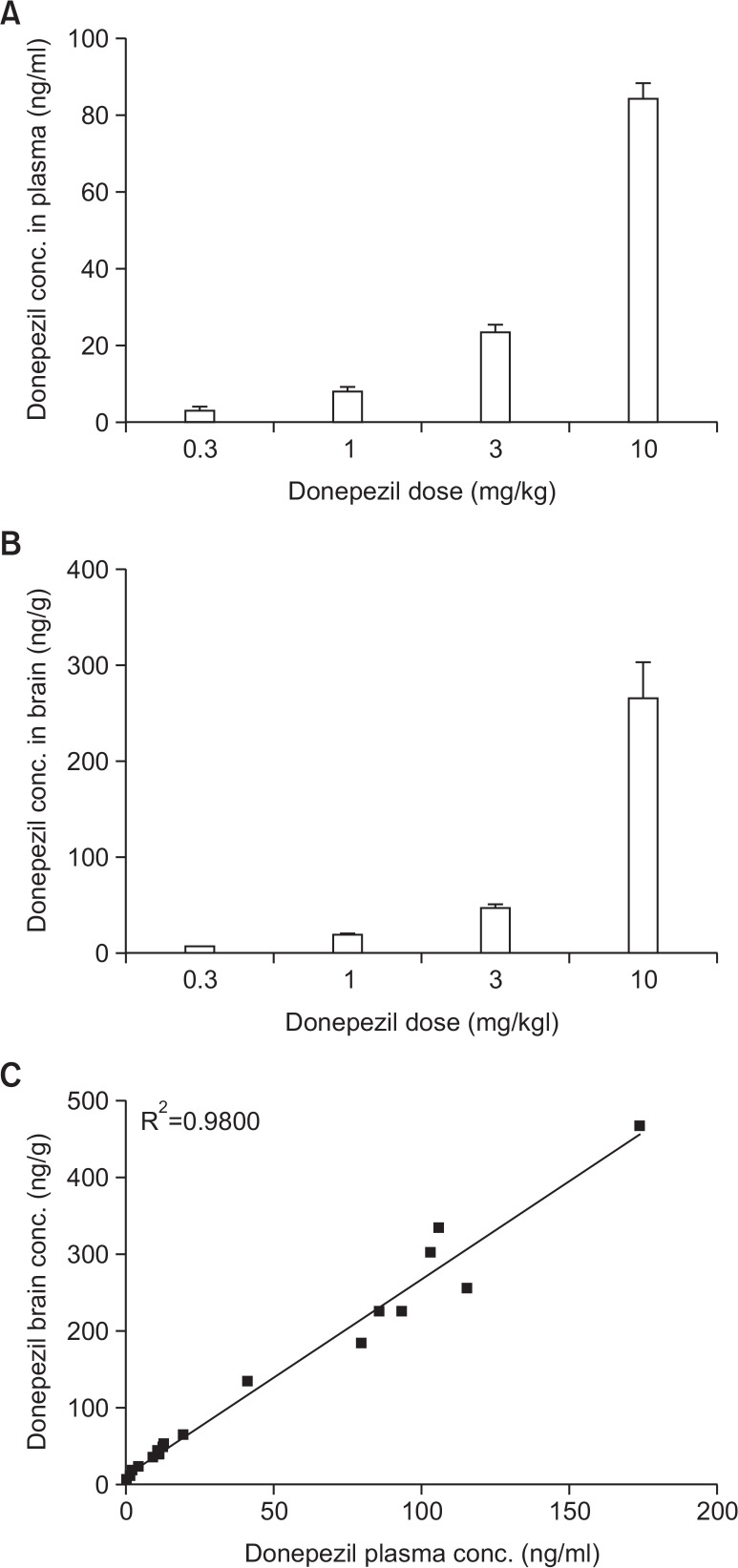
To explore the effective concentration of donepezil for improving learning and memory, plasma or brain levels of donepezil were determined. Donepezil HCl (0.3–10 mg/kg free drug dissolved in saline) was administered orally for four consecutive days to the corresponding treatment groups (8 animals per group). Plasma and mouse brains were then isolated, and the concentration of donepezil was measured 1 h after the fourth dose of donepezil HCl. Fig. 7 show dose-dependent changes in the plasma (Fig. 7A) and brain (Fig. 7B) concentration of donepezil in mice. After oral treatment, the concentration of donepezil in the brain was higher than that in the plasma; brain donepezil levels positively correlated with plasma donepezil levels (Pearson’s correlation coefficient R2=0.9800, p<0.001) (Fig. 7C). These results suggest that donepezil has good brain penetration.
Fig. 7.
Relationship between brain and plasma donepezil concentrations and the pharmacological effects. Donepezil HCl (0.3–10 mg/kg free drug dissolved in saline) was administered orally for four consecutive days to the corresponding treatment groups (8 animals per group). Dose-dependent changes in plasma (A) and brain (B) concentrations of donepezil were observed 1 h after the fourth dose of donepezil HCl in mice. After oral treatment, brain donepezil levels positively correlated with plasma donepezil levels (C). Data represent the means ± SEM.
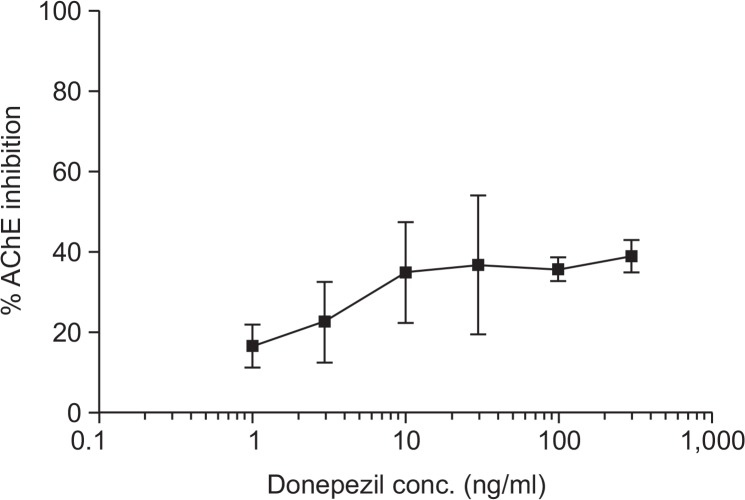
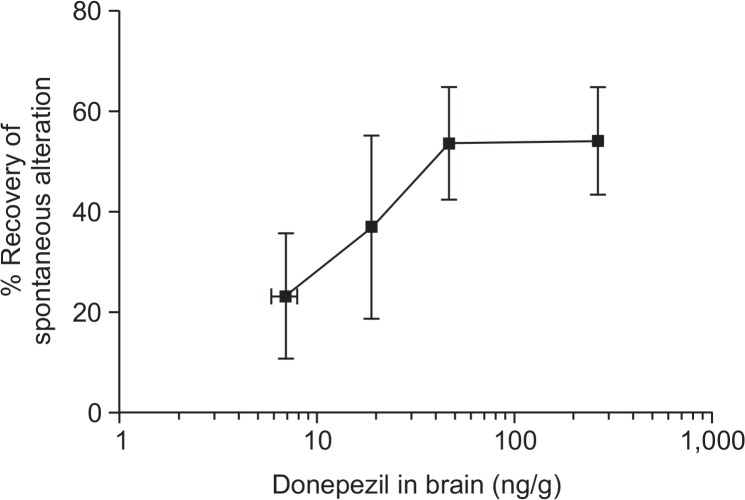
Next, we performed PK/PD analyses between brain levels of donepezil and recovery of scopolamine-induced learning and memory impairment in mice to predict the effective brain concentration of donepezil. As the concentration of donepezil in the brain increased, the recovery of SAs also improved; maximal improvement was observed at 46.5 ± 3.5 ng/g (equivalent to 3 mg/kg dose) (Fig. 8). These results show that donepezil in the brain improves memory impairment in a concentration-dependent manner; however, there is a limit to the recovery effects of donepezil.
Fig. 8.
Relationship between the brain concentration of donepezil and the pharmacological effects. Donepezil HCl (0.3–10 mg/kg free drug dissolved in saline) was administered orally for four consecutive days to the corresponding treatment groups (8 animals per group). As the concentration of donepezil in the brain increased, the recovery of spontaneous alternations (SA) also improved. Data represent the means ± SEM.
DISCUSSION
In this study, we characterized the effective concentration of donepezil for AChE inhibition and impaired learning and memory according to a PK and PD assay in rodents.
The data suggest that AChE activity is a sensitive biomarker that positively correlates with donepezil plasma concentrations. Furthermore, we demonstrated that donepezil, a novel AChE inhibitor, attenuates scopolamine-induced learning and memory impairment in a manner that positively correlates with the concentration of donepezil measured in the brain.
In this study, we delineated the PK characteristics of hairless rats. Although there have been several reports regarding the PK profiles of donepezil after oral (Matsui et al., 1999; Goh et al., 2011) or intravenous administration (Saluja et al., 2013) in rodents, the absolute bioavailability (F) following oral administration remained unclear. In this study, a PK analysis showed low absolute bioavailability of donepezil; this result differs from that in a previous report (Goh et al., 2011). However, the higher bioavailability (30–40%) of donepezil reported by (Goh et al., 2011) followed a 1-h IV infusion of donepezil; this difference in IV dosage regimens may explain the conflicting results. In this study, a bolus IV dose of 3 mg/kg was administered to hairless rats, and this dose was selected based on a reported lethal dose of 7.7 mg/kg in rats (Saluja et al., 2013).
To measure PD response to AChE inhibition, an AChE activity assay was performed using rat plasma. Although inhibition of AChE activity is dependent on the concentration of donepezil, the basal AChE activity in rat plasma varies and likely affects maximal % inhibition. In this study, the maximal inhibition achieved after donepezil treatment was approximately 30%. Inhibition of AChE was positively correlated with the concentration of donepezil in plasma (R2=0.5099). The maximal PD effects of donepezil in plasma (approximately 40% inhibition) have been shown to occur at a range of 30–100 ng/ml donepezil ex vivo. In the current study, donepezil-induced inhibition of AChE was less potent in the rat plasma, an effect that agrees with the results of Haug et al. (2005). These data suggested that donepezil does not completely inhibit rat plasma cholinesterase, attributable to the presence of both AChE and butyrylcholinesterase (Traina and Serpietri, 1984; Kosasa et al., 2000).
The most frequently observed adverse events (AEs) associated with donepezil include cholinergic effects related to the gastrointestinal system (nausea, vomiting, and diarrhea) and sleep disorders-the frequency and severity of which depend on the chosen dose (Seltzer, 2007; Farlow et al., 2011). The incidence of AEs increased with oral administration, attributable to large and frequent plasma fluctuations (Sozio et al., 2012). To decrease plasma fluctuations and attenuate the AEs, control of donepezil levels in the plasma and brain is important.
SAs are a measure of exploration behavior in mice, and measurement of SAs is a reliable screening model to test the effects of drugs against scopolamine-induced memory impairments (Sarter et al., 1988). A mouse with an impaired short-term memory cannot recall which arm it has just visited and thus tends to exhibit decreased SAs (Wietrzych et al., 2005). However, there are discrepancies between the dose of donepezil reported to be effective in learning and memory studies (Kosasa et al., 2000). The Y-maze test is used to evaluate attention and short-term working memory of rodents. It is based on the propensity of rodents to explore new environments. The indicator of short-term working memory in this test is SA behavior (Yang et al., 2009). In this test, treatment with scopolamine, an acetylcholine receptor antagonist known to produce memory deficits, impairs SA behavior in mice (Olton and Papas, 1979; Dela Pena et al., 2017). In the present study, we measured SA behavior in the Y-maze test to predict the effective dose of donepezil. Results of the present study showed that scopolamine produces a decrease in alternation behavior of mice using the Y-maze paradigm, indicating memory impairment. This finding is in agreement with previous studies (Mugwagwa et al., 2015). Behavioral studies have shown that pretreatment with donepezil with a dose ranging from 3 to 10 mg/kg significantly ameliorates performance deficits in several learning and memory tasks in mice after scopolamine treatment.
As the relation between PK and PD is usually addressed by measuring plasma or brain donepezil levels, we measured both plasma and brain donepezil levels in mice after conducting the behavioral studies. Donepezil levels were increased in both plasma and brain tissues with increasing doses, and brain donepezil levels were positively correlated with plasma donepezil levels. The tissue to plasma (T/P) ratio showed an excellent correlation (R2=0.9800). A previous non-clinical study suggested that the brain to plasma ratio for donepezil ranges from 3.3 to 5.2 in mice (Geerts et al., 2005). Consistent with these results, donepezil concentrations in the brain were 3- to 4-fold higher than those in the plasma and may be mediated by organic cation transporters (Kim et al., 2010).
Based on the hypothesis that donepezil-induced AChE inhibition in the blood corresponds closely to its effects in the cerebral cortex of rats (Rogers et al., 1998), we investigated the association between donepezil levels in the brain and primary efficacy outcomes such as learning and memory impairment. In the present study, % recovery of alternations improved with increasing concentrations of donepezil in the brain, and maximal effects were observed at 46.5 ± 3.5 ng/g donepezil in the brain; however, impaired learning and memory were not fully recovered. These results support the idea that the ameliorating effect of donepezil on performance deficits in several learning and memory tasks in mice is based upon donepezil levels in the brain.
Although randomized and controlled studies are required to define the effective concentration of donepezil in the brain, when taken together, our findings indicate that orally administered donepezil penetrates the brain and improves impaired learning and memory.
Acknowledgments
This research was supported by the Senior-friendly Product R&D program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C1960).
Footnotes
CONFLICT OF INTEREST
The author retains the right to provide an electronic copy of the final peer-reviewed manuscript to Korea Pubmed Center (PMC) upon acceptance for Journal publication and make it publicly available as soon as possible, but no later than 12 months after publication by the Journal.
REFERENCES
- Bhateria M, Ramakrishna R, Pakala DB, Bhatta RS. Development of an LC-MS/MS method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1001:131–139. doi: 10.1016/j.jchromb.2015.07.042. [DOI] [PubMed] [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Doyle PD, Carroll RT, Emmerling MR, Jaen J. Cholinergic therapies for Alzheimer’s disease. Palliative or disease altering? Arzneimittelforschung. 1995;45:425–431. [PubMed] [Google Scholar]
- Dela Pena IJI, Kim HJ, Botanas CJ, de la Pena JB, Van Le TH, Nguyen MD, Park JH, Cheong JH. The psychopharmacological activities of Vietnamese ginseng in mice: characterization of its psychomotor, sedative-hypnotic, antistress, anxiolytic, and cognitive effects. J Ginseng Res. 2017;41:201–208. doi: 10.1016/j.jgr.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M, Veloso F, Moline M, Yardley J, Brand-Schieber E, Bibbiani F, Zou H, Hsu T, Satlin A. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer’s disease. BMC Neurol. 2011;11:57. doi: 10.1186/1471-2377-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Goh CW, Aw CC, Lee JH, Chen CP, Browne ER. Pharmacokinetic and pharmacodynamic properties of cholinesterase inhibitors donepezil, tacrine, and galantamine in aged and young Lister hooded rats. Drug Metab Dispos. 2011;39:402–411. doi: 10.1124/dmd.110.035964. [DOI] [PubMed] [Google Scholar]
- Haug KH, Bogen IL, Osmundsen H, Walaas I, Fonnum F. Effects on cholinergic markers in rat brain and blood after short and prolonged administration of donepezil. Neurochem Res. 2005;30:1511–1520. doi: 10.1007/s11064-005-8828-6. [DOI] [PubMed] [Google Scholar]
- Ito Y, Harada T, Fushimi K, Kagawa Y, Oka H, Nakazawa H, Homma R, Kato Y, Yamada S. Pharmacokinetic and pharmacodynamic analysis of acetylcholinesterase inhibition by distigmine bromide in rats. Drug Metab Pharmacokinet. 2010;25:254–261. doi: 10.2133/dmpk.25.254. [DOI] [PubMed] [Google Scholar]
- Kim MH, Maeng HJ, Yu KH, Lee KR, Tsuruo T, Kim DD, Shim CK, Chung SJ. Evidence of carrier-mediated transport in the penetration of donepezil into the rat brain. J Pharm Sci. 2010;99:1548–1566. doi: 10.1002/jps.21895. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Inhibitory effect of orally administered donepezil hydrochloride (E2020), a novel treatment for Alzheimer’s disease, on cholinesterase activity in rats. Eur J Pharmacol. 2000;389:173–179. doi: 10.1016/S0014-2999(99)00876-6. [DOI] [PubMed] [Google Scholar]
- Matsui K, Mishima M, Nagai Y, Yuzuriha T, Yoshimura T. Absorption, distribution, metabolism, and excretion of donepezil (Aricept) after a single oral administration to Rat. Drug Metab Dispos. 1999;27:1406–1414. [PubMed] [Google Scholar]
- Moon M, Song H, Hong HJ, Nam DW, Cha MY, Oh MS, Yu J, Ryu H, Mook-Jung I. Vitamin D-binding protein interacts with Aβ and suppresses Aβ-mediated pathology. Cell Death Differ. 2013;20:630–638. doi: 10.1038/cdd.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugwagwa AT, Gadaga LL, Pote W, Tagwireyi D. Antiamnesic effects of a hydroethanolic extract of Crinum macowanii on scopolamine-induced memory impairment in mice. J Neurodegener Dis. 2015;2015:242505. doi: 10.1155/2015/242505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- Saluja S, Kasha PC, Paturi J, Anderson C, Morris R, Banga AK. A novel electronic skin patch for delivery and pharmacokinetic evaluation of donepezil following transdermal iontophoresis. Int J Pharm. 2013;453:395–399. doi: 10.1016/j.ijpharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology (Berl) 1988;94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- Seltzer B. Donepezil: an update. Expert Opin Pharmacother. 2007;8:1011–1023. doi: 10.1517/14656566.8.7.1011. [DOI] [PubMed] [Google Scholar]
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. doi: 10.1001/jama.1997.03550160083043. [DOI] [PubMed] [Google Scholar]
- Sozio P, Cerasa LS, Marinelli L, Di Stefano A. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr. Dis Treat. 2012;8:361–368. doi: 10.2147/NDT.S16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramek JJ, Cutler NR. RBC cholinesterase inhibition: a useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis. Assoc. Disord. 2000;14:216–227. doi: 10.1097/00002093-200010000-00006. [DOI] [PubMed] [Google Scholar]
- Traina ME, Serpietri LA. Changes in the levels and forms of rat plasma cholinesterases during chronic diisopropylphosphorofluoridate intoxication. Biochem Pharmacol. 1984;33:645–653. doi: 10.1016/0006-2952(84)90321-6. [DOI] [PubMed] [Google Scholar]
- Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, Chambon P, Krezel W. Working memory deficits in retinoid X receptor gamma-deficient mice. Learn Mem. 2005;12:318–326. doi: 10.1101/lm.89805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Han SJ, Ryu JH, Jang IS, Kim DH. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol Pharm Bull. 2009;32:1710–1715. doi: 10.1248/bpb.32.1710. [DOI] [PubMed] [Google Scholar]