ABSTRACT
Stress can impair protein folding in the endoplasmic reticulum (ER). Minimizing the accumulation of misfolded proteins in the ER is achieved by ER-associated degradation (ERAD), which involves the retrograde transport and proteasomal removal of aberrant proteins. Recently, the proteasome has been implicated in a selenium stress response. However, it remains unknown if selenium causes ER stress in plants similar to animals, and if ERAD is associated with optimal selenium tolerance. This deficiency was addressed by monitoring selenate-treated Arabidopsis plants with mutations in HRD1 and SeL1L, participants of ERAD. hrd1a/hrd1b and sel1l mutants treated with selenate demonstrate decreased tolerance and ER stress, as judged by BiP2 accumulation. The data indicate that optimal plant growth during selenate stress requires ERAD.
KEYWORDS: BiP2, ER, ERAD, protein misfolding, selenium, oxidative stress, UPR
Abbreviations
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum associated degradation
- UPR
unfolded protein response
- ROS
reactive oxygen species
- Se
selenium
- WT
wildtype
Protein quality control is integral to cellular survival, but a variety of abiotic stressors, including heat and salt, can endanger protein structure and consequently lead to misfolding in cells. This is particularly salient in the endoplasmic reticulum (ER) where nascent polypeptides obtain their native confirmation and modifications- including glycosylation- prior to secretion.1,2 Even though it is estimated that one-third of newly synthesized proteins are misfolded during ambient conditions,3 ER homeostasis in eukaryotes is maintained by ER-associated degradation (ERAD) of non-native proteins.4,5 This form of protein quality control in the ER involves the retrograde transport of misfolded proteins into the cytosol, and their subsequent ubiquitination and degradation by the 26S proteasome. Therefore, ERAD prevents misfolded proteins from forming toxic protein aggregates, thereby averting ER stress.
However, ER poise can be disturbed by stressors that overwhelm ERAD's capacity to clear misfolded proteins.2 For example, tunicamycin and dithiothreitol both result in ER stress by inhibiting glycosylation and reducing disulfide bridges in proteins, respectively. An accumulation of reactive oxygen species (ROS) can also lead to protein misfolding and ER stress.6,7 In addition to chemicals and abiotic stress, ER stress can be triggered by genetic defects in ERAD, causing an accumulation of unfolded proteins in the ER lumen.8,9 In each of the cases above, ER stress elicits an unfolded protein response (UPR), which serves to refold proteins by chaperone-mediated processes or degrade irreparable proteins by the ubiquitin-proteasome pathway.10,11 Additionally, UPR decreases de novo protein synthesis to avert further accumulation of misfolded proteins.
Although ERAD is best characterized in yeast, this proteolytic pathway connecting the ER and cytosol also occurs in plants.4 The recent discovery of HRD1, HRD3A, and OS9 proteins in Arabidopsis indicates that plants and yeast use similarly ERAD machinery, as reviewed.9 The HRD complex resides on the ER membrane, and is responsible for removing proteins with folding defects exposed to the ER membrane or lumen. (A separate DOA10 complex in yeast removes incorrectly folded proteins facing the cytosol, but is uncharacterized in plants despite the discovery of homologous components).12 Functionally redundant HRD1A and HRD1B are E3 ubiquitin ligases that assist in protein ubiquitination prior to delivery to the 26S proteasomes.13 Another component of the HRD complex is HRD3A, the only homolog of yeast HRD3 and mammalian SEL1L.14 HRD3A and the luminal lectin OS9 are believed to work together to recognize and recruit misfolded proteins to the HRD complex,7 where aberrant proteins are ultimately retrotranslocated to the proteasome.
The integrity of the HRD complex is associated with stress tolerance in Arabidopsis. Double knockout of HRD1A and HRD1B results in elevated salt sensitivity in hrd1a/hrd1b Arabidopsis plants, which was explained by NaCl-induced ROS accumulation that affected the formation of disulfide bridges.9 Additionally, mutation in HRD3A renders Arabidopsis plants sensitive to salt and tunicamycin. Both stressors induced expression of BIP1/2, which encodes an ER chaperone involved in UPR; however, expression was greater in hrd3a plants compared to wildtype plants, suggesting that ER stress was exacerbated in the mutants.14
Whether or not optimal selenium (Se) stress also requires ERAD is unknown. Although plants can accumulate Se, it is not required in higher plants as it is in mammals.15 Se generates ROS, and its toxicity in plants can partially be attributed to oxidative stress.16-18 Selenite treatment rapidly induces mitochondrial superoxide in the green algae Chlamydomonas.19 This type of ROS was also observed in selenite-treated Brassica napus, and coincided with altered respiration and a mitochondrial stress response.20 Inorganic Se can be assimilated into Se-cysteine via the sulfur assimilatory pathway.21 The inadvertent replacement of cysteine with selenocysteine in proteins has long been associated with misfolding, and assumed to be another form of Se toxicity.22,23 Recently, the proteasome has been implicated in the removal of malformed selenoproteins in plants, and supports the misfolded selenoprotein hypothesis. Proteasome inhibition in Se-treated Stanleya pinnata24 and Chlamydomonas19 increases the amount of Se in protein, indicating that functioning proteasomes act to degrade nonspecific selenoproteins. Additionally, Se-cysteine increases proteasome activity in B. napus,25 further suggesting that a cysteine to Se-cysteine substitution is toxic. Coinciding with the above data, Arabidopsis mutants with impaired 26S proteasome activity are sensitive to selenate.24
The aim of this study was to determine if defects in ERAD would decrease selenate tolerance in Arabidopsis. ERAD mutant plants used in this study were obtained as hrd1a/hrd1b9 and sel1l14 seeds, and compared to wildtype (WT) plants in the same genetic background (ecotype Columbia). Plants were grown in a growth chamber (150 microEinstein, 16 h light/8 h dark cycle, 24°C) on vertical agar plates containing 0.5 strength MS media with or without 40 μM sodium selenate, a concentration known to induce Se stress.26 To determine selenate toxicity, plants (n = 30) were grown with and without selenate. Selenate tolerance was determined by measuring root length in the WT and mutant plants after 10 d. All statistical analyses (ANOVA) were performed using the Kaleida-graph software package (Synergy Software).
There was no difference in plant growth, as determined by measuring root lengths, among the 3 ecotypes on control media as observed earlier.9,14 Compared to control media, root length in WT plants challenged with selenate decreased by 40%, demonstrating its toxicity (Fig. 1a). Defects in ERAD exacerbated selenate's toxicity. Compared to WT plants treated with selenate, root length of sel1l and hrd1a/hrd1b decreased by 40% and 50%, respectively. These data suggest that maintenance of ERAD is required for optimal tolerance to selenate.
Figure 1.
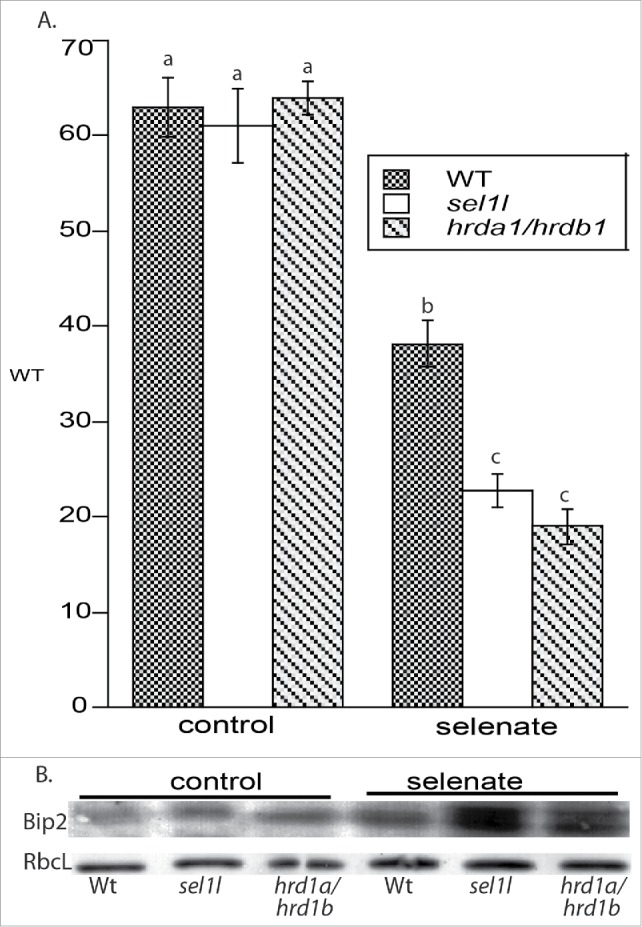
(a) sel1l and hrd1a/hrd1b plants have decreased tolerance to selenate. Plants were grown on 0.5MS vertical agar plants with or without 40 μM selenate. After 10 d, root lengths were measured. Lowercase letters represent a significant difference between treatments (p < 0.05, n = 30 replicates). Shown are the mean and SE, and represent another experimental replicate. (b) ERAD mutants accumulate the ER chaperone BiP2 on media with selenate for 10 d. Immunoreactive bands represent 4 other experimental replicates. RbcL- large subunit of RUBISCO
To determine if Se induces ER stress in WT and ERAD mutants, levels of Bip2 in seedlings were analyzed on a 15% SDS-PAGE gel as previously described.20 This ER chaperone accumulates in response to stressors that trigger UPR2,4. In untreated plants, levels of Bip2 accumulate in ERAD mutants compared to WT. Although Se treatment increases Bip2 levels in WT plants, its accumulation is even more pronounced in the ERAD mutants treated with selenate (Fig. 1b); this result coincides with Bip2s accumulation in hrd3a plants treated with salt and tunicamycin.14 The presence of 2 bands in sel1l and hrd1a/hrd1b plants challenged with selenate probably reflects nonspecific immunoreactivity of the Bip2 antibody against either Bip1 or Bip3 . Levels of the large subunit of RUBICSO (RbcL) were not altered.
Increased levels of BiP2 indicate that selenate induces ER stress during ERAD impairment, which likely explains the elevated sensitivity of the ERAD mutants to Se stress, as depicted in a schematic model (Fig. 2). Selenate sensitivity was also observed in Arabidopsis plants with impaired proteasome activity. Intriguingly, however, selenate did not affect bip2-1 Arabidopsis plants.24 This would suggest that Se toxicity does not induce ER stress if intact ERAD machinery can remove damaged proteins from the ER, and deliver aberrant proteins to the proteasome. Computational and manual analysis of transcriptome data26 further suggest that selenate does not induce expression of common UPR or ER stress-related transcripts.4
Figure 2.
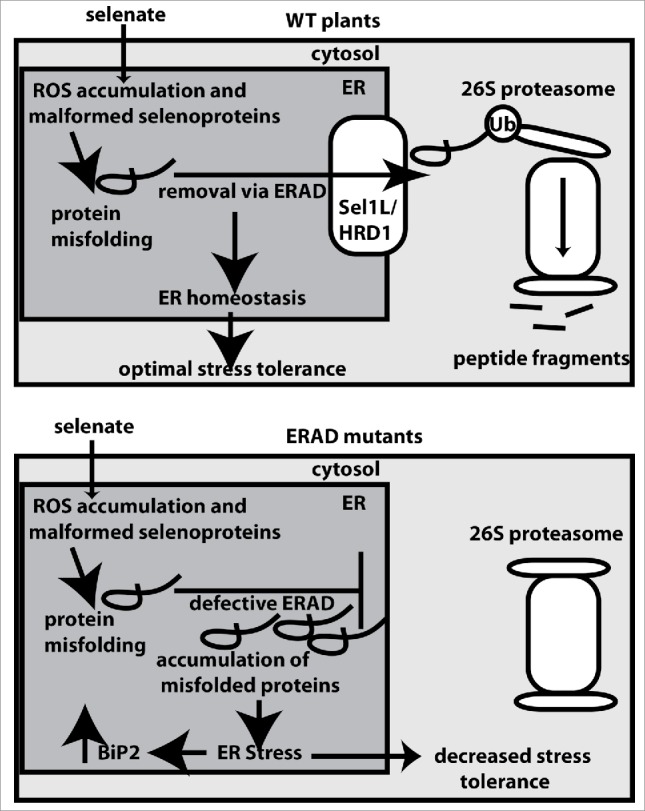
Schematic model depicting Arabidopsis' response to selenate in wildtype (top) and ERAD mutant plants (bottom). In wildtype plants, ERAD removes selenate-induced misfolded proteins from the ER to the cytosol, where the ubiquitinated proteins are degraded by the 26S proteasome. sel1l and hrd1a/hrd1b plants have defects in ERAD machinery. As a result, misfolded proteins accumulate in the ER, which triggers UPR and increased levels of BiP2. ER stress in the ERAD mutants likely contributes to decreased selenate tolerance. Ub- ubiquitin
Because Se toxicity causes both oxidative stress and most likely protein misfolding as a result of a cysteine to Se-cysteine substitution,18 it is difficult to ascertain why selenate directly caused decreased growth and ER stress in the ERAD mutants. Yeast with defective ERAD machinery display ER stress and sensitivity to canavanine, a structural analog of arginine.8 Therefore, it is feasible that cysteine's analog Se-cysteine may also cause ER stress in ERAD mutants. In human cells, Se caused an ER stress response, but the accumulation of unfolded proteins in the ER was attributed to Se-induced oxidative stress and altered redox status.27 In this context, it is worth noting that selenate can deplete the pool of reduced glutathione in Arabidopsis which can perturb redox poise.17,28
In conclusion, optimal selenate tolerance in Arabidopsis is dependent upon intactness of the HRD complex, a component of ERAD. Whether or not overexpression of genes participating in ERAD pathway confer Se tolerance is unknown. Because Se is an essential trace element and has purported health benefits in humans, plants with fortified levels of Se and enhanced tolerance have been envisioned to have therapeutic applications.15 This goal might be achieved in plants with enhanced ERAD efficiency, but this remains to be experimentally tested.
Funding Statement
NSF | BIO | Division of Molecular and Cellular Biosciences (MCB) MCB-1244009
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
DVH is indebted to Richard Strasser for providing the sel1l and hrd1a/hrd1b seeds used in this study. This project was supported from the NSF-RUI Program (MCB-1244009).
References
- 1.Staehelin LA. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 1997; 11:1151-65; PMID:9225461; https://doi.org/ 10.1046/j.1365-313X.1997.11061151.x [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 2004; 14:20-8; PMID:14729177; https://doi.org/ 10.1016/j.tcb.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 2000; 404:770-4; PMID:10783891; https://doi.org/ 10.1038/35004754 [DOI] [PubMed] [Google Scholar]
- 4.Liu JX, Howell SH. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010; 22:2930-42; PMID:20876830; https://doi.org/ 10.1105/tpc.110.078154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vembar SS, Brodsky JL. One step at a time: Endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 2008; 9:944-57; PMID:19002207; https://doi.org/ 10.1038/nrm2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura M, Hiramatsu N. The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. Biometals 2010; 23:941-50; PMID:20130962; https://doi.org/ 10.1007/s10534-010-9296-2 [DOI] [PubMed] [Google Scholar]
- 7.Hüttner S, Veit C, Schoberer J, Grass J, Strasser R. Unraveling the function of Arabidopsis thaliana OS9 in the endoplasmic reticulum-associated degradation of glycoproteins. Plant Mol Biol 2012; 79:21-33; PMID:22328055; https://doi.org/ 10.1007/s11103-012-9891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Chang A. Substrate recognition in ER‐associated degradation mediated by Eps1, a member of the protein disulfide isomerase family. EMBO J 2003; 22:3792-802; PMID:12881414; https://doi.org/ 10.1093/emboj/cdg378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hüttner S, Strasser R. Endoplasmic reticulum-associated degradation of glycoproteins in plants. Front Plant Sci 2012; 3:67; PMID:22645596; https://doi.org/10.3389/fpls.2012.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev Mol Cell Biol 2007; 8:519-29; PMID:17565364; https://doi.org/ 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 11.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2005; 6:29-63; https://doi.org/ 10.1016/j.mrfmmm.2004.06.056 [DOI] [PubMed] [Google Scholar]
- 12.Müller J, Piffanelli P, Devoto A, Miklis M, Elliott C, Ortmann B, Schulze-Lefert P, Panstruga R. Conserved ERAD-like quality control of a plant polytopic membrane protein. Plant Cell 2005; 17:149-163; PMID:15598804; https://doi.org/ 10.1105/tpc.104.026625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su W, Liu Y, Xia Y, Hong Z, Li J. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. PNAS 2011; 108:870-875; PMID:21187394; https://doi.org/ 10.1073/pnas.1013251108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Cui F, Li Q, Yin B, Zhang H, Lin B, Wu Y, Xia R, Tang S, Xie Q. The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Research 2011; 21:957-969; PMID:21187857; https://doi.org/ 10.1038/cr.2010.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 2009; 14:436-42; PMID:19665422; https://doi.org/ 10.1016/j.tplants.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Rad Biol Med 1994; 17:45-64; PMID:7959166; https://doi.org/ 10.1016/0891-5849(94)90007-8 [DOI] [PubMed] [Google Scholar]
- 17.Hugouvieux V, Dutilleul C, Jourdain A, Reynaud F, Lopez V, Bourguignon J. Arabidopsis putative selenium-binding protein1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiol 2009; 151:768-81; PMID:19710230; https://doi.org/ 10.1104/pp.109.144808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hoewyk D. A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annals Bot 2013; 112:965-72; PMID:23904445; https://doi.org/ 10.1093/aob/mct163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallentine P, Hung CY, Xie J, Van Hoewyk D. The ubiquitin–proteasome pathway protects Chlamydomonas reinhardtii against selenite toxicity, but is impaired as reactive oxygen species accumulate. AoB Plants 2014; 6:plu062; PMID:25301821; https://doi.org/10.1093/aobpla/plu062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimkovikj A, Van Hoewyk D. Selenite activates the alternative oxidase pathway and alters primary metabolism in Brassica napus roots: evidence of a mitochondrial stress response. BMC Plant Biology 2014; 14:259; PMID:25267309; https://doi.org/ 10.1186/s12870-014-0259-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilon-Smits EAH, Quinn CF. 2010. Selenium metabolism in plants In: Hell R, Mendel R eds. Cell biology of metal and nutrients. Berlin: Springer, pp. 225-41. [Google Scholar]
- 22.Brown TA, Shrift A. Exclusion of selenium from proteins of selenium tolerant Astragalus species. Plant Physiol 1981; 67:1051-3; PMID:16661781; https://doi.org/ 10.1104/pp.67.5.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhierl B, Bock A. On the mechanism of selenium tolerance in selenium- accumulating plants. Purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisulcatus. Euro J Biochem 1996; 239:235-8; PMID:8706715; https://doi.org/ 10.1111/j.1432-1033.1996.0235u.x [DOI] [PubMed] [Google Scholar]
- 24.Sabbagh M, Van Hoewyk D. Malformed selenoproteins are removed by the ubiquitin- proteasome pathway in Stanleya pinnata. Plant Cell Physiol 2012; 53:555-64; PMID:22323770; https://doi.org/ 10.1093/pcp/pcs015 [DOI] [PubMed] [Google Scholar]
- 25.Dimkovikj A, Fisher B, Hutchison K, Van Hoewyk D. Stuck between a ROS and a hard place: Analysis of the ubiquitin proteasome pathway in selenocysteine treated Brassica napus reveals different toxicities during selenium assimilation. J Plant Physiol 2015; 181:50-4; PMID:25974369; https://doi.org/ 10.1016/j.jplph.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EAH. Transcriptome analyses give insight into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plantarum 2008; 132:236-53; https://doi.org/10.1111/j.1399-3054.2007.01002.x [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res 2005; 65:9073-9; PMID:16204082; https://doi.org/ 10.1158/0008-5472.CAN-05-2016 [DOI] [PubMed] [Google Scholar]
- 28.Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH: Glutathione in plants: an integrated overview. Plant Cell Environ 2012; 35:454-84; PMID:21777251; https://doi.org/ 10.1111/j.1365-3040.2011.02400.x [DOI] [PubMed] [Google Scholar]


