Abstract
Aims/Introduction
Whereas some clinical studies have shown that excessive fat accumulation in the pancreas is associated with impairment of insulin secretion, others have not found such an association. 1H magnetic resonance spectroscopy allows quantitative fat analysis in various tissues including the pancreas. The pathological relevance of pancreatic fat content (PFC) in Japanese individuals remains unclear, however.
Materials and Methods
We analyzed PFC in 30 Japanese individuals with normal glucose tolerance by 1H magnetic resonance spectroscopy, and then investigated the relationships between PFC and indexes of insulin secretion and insulin sensitivity‐resistance determined by an oral glucose tolerance test. We also measured hepatic fat content and intramyocellular lipid content by 1H magnetic resonance spectroscopy, as well as visceral fat area and subcutaneous fat area by magnetic resonance imaging, and we examined the relationships between these fat content measures and oral glucose tolerance test‐derived parameters.
Results
PFC was correlated with indexes of insulin sensitivity‐resistance, but not with those of insulin secretion. Hepatic fat content and visceral fat area were correlated with similar sets of parameters as was PFC, whereas subcutaneous fat area was correlated with parameters of insulin secretion, and intramyocellular lipid content was not correlated with any of the measured parameters. The correlation between PFC and homeostasis model assessment of insulin resistance remained significant after adjustment for age, body mass index and sex. Among fat content measures, PFC was most highly correlated with hepatic fat content and visceral fat area.
Conclusions
PFC was correlated with indexes of insulin resistance, but not with those of insulin secretion in non‐obese Japanese individuals with normal glucose tolerance.
Keywords: Insulin resistance, Insulin secretion, Pancreatic fat
Introduction
Obesity is the most common and important contributing factor to the development of insulin resistance and consequent pathological disorders including type 2 diabetes mellitus. Evidence suggests that the exaggerated accumulation of fat in non‐adipose insulin‐sensitive tissues – that is, skeletal muscle and liver – is associated with insulin resistance1, 2, and that certain lipids impair the effects of insulin both in cells and in the living body3. Excess fat in these non‐adipose tissues is thus thought to contribute to the pathogenesis of insulin resistance induced by obesity.
The accumulation of fat also occurs in the pancreas4, 5. Evidence obtained from cell culture or animal studies suggests that excess fat inhibits insulin secretion or accelerates apoptosis in pancreatic β‐cells6, 7, effects that also might be related to the pathogenesis of type 2 diabetes. Some clinical studies support the notion that pancreatic fat content (PFC) is negatively correlated with insulin secretion or β‐cell function8, 9, 10, whereas others have not detected such a correlation11, 12, 13.
The impact of fat accumulation on the development of metabolic disorders is thought to differ among ethnicities14. Although the extent and prevalence of obesity are much lower in East Asian countries including Japan than in the USA and Europe15, the prevalence of obesity‐related diseases, such as type 2 diabetes or non‐alcoholic fatty liver disease, does not show such a difference16, 17. Given that relatively lower levels of fat accumulation likely elicit greater effects on the development of metabolic disorders in Japanese individuals, the definition of obesity in Japan (a body mass index [BMI] of >25 kg/m2)18 differs from that in the USA and European countries (BMI of >30 kg/m2)19, 20. However, information regarding the pathological relevance of PFC in Japanese people is limited. Pancreatic fat as assessed by computed tomography (CT) was shown to be correlated with impaired insulin secretion21 or to be independent of the incidence of type 2 diabetes mellitus22 in Japanese people.
PFC can be evaluated by several non‐invasive techniques including CT, ultrasonography, magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS)4, 5. PFC assessed by 1H‐MRS has been shown to be highly concordant with that determined by biochemical analysis of tissue homogenates23. In addition, given that similar patterns of fat accumulation have been observed in the exocrine and endocrine pancreas of obese animals24, PFC analysis by 1H‐MRS is thought to provide a good quantitative surrogate of fat accumulation in pancreatic islets4, 5. PFC has not previously been evaluated by 1H‐MRS in the Japanese population, however.
To provide further insight into the physiological relevance of excess fat in the pancreas, we have now measured PFC with the use of 1H‐MRS in Japanese individuals, and then examined the relationships between PFC and parameters of insulin secretion or insulin sensitivity‐resistance determined by an oral glucose tolerance test (OGTT). To avoid secondary effects of treatment or hyperglycemia, we recruited volunteers with normal glucose tolerance (NGT) as study participants. We also measured fat accumulation in the liver and skeletal muscle, as well as the areas of subcutaneous and visceral fat tissues with 1H‐MRS or MRI, respectively. We found that PFC was correlated with parameters of insulin sensitivity‐resistance, but not with those of insulin secretion, as well as with hepatic and visceral fat accumulation in Japanese individuals with NGT.
Methods
Study participants
The study was approved by the ethics committee of Kobe University Graduate School of Medicine, conforms to the provisions of the Declaration of Helsinki (as revised in 2013) and is registered in the University Hospital Medical Information Network (UMIN000003753). A total of 43 volunteers who had not been diagnosed with diabetes mellitus and whose first‐degree relatives had no history of this disease were recruited at the Division of Diabetes and Endocrinology of Kobe University Hospital from January 2011 to August 2013. All these individuals provided written informed consent to participate in the study. They underwent a 75‐g OGTT and comprehensive fat content analysis with the use of consecutive 1H‐MRS and MRI within a period of 2 weeks. Of the 43 individuals, 13 were found to manifest either impaired glucose tolerance (plasma glucose level at 120 min after initiation of the OGTT of ≥140 mg/dL and <200 mg/dL) or impaired fasting glycemia (fasting plasma glucose level of ≥110 mg/dL and <126 mg/dL). We therefore included the 30 individuals with NGT (fasting plasma glucose of 110 mg/dL and plasma glucose level at 120 min after initiation of the OGTT of <140 mg/dL) as study participants for further analysis.
OGTT‐based clinical parameters
A standard 75‐g OGTT was carried out in the morning after the participants had fasted overnight. Blood samples were collected before, and at 30, 60, 90, and 120 min after ingestion of glucose for the measurement of plasma glucose and serum insulin concentrations. For the assessment of insulin secretion, we calculated the insulinogenic index, homeostasis model assessment of β‐cell function (HOMA‐β), the area under the curve for serum insulin concentration (AUCins120) and the ratio of the area under the curve for serum insulin concentration to that for plasma glucose concentration (AUCins/glu120). The insulinogenic index was calculated as the change in serum insulin concentration divided by that in plasma glucose concentration from 0 to 30 min during the OGTT. For the assessment of insulin sensitivity‐resistance, we calculated the composite index25 and homeostasis model assessment of insulin resistance (HOMA‐IR). An OGTT‐based analog of the disposition index (DI), which we termed the oral DI, was calculated as the product of the composite index and AUCins/glu120, as described previously26.
Comprehensive fat content analysis by 1H‐MRS and MRI
For fat content analysis, magnetic resonance scanning was carried out in the afternoon with participants in the non‐fasted state and with the use of a 3.0‐T whole‐body system (Achieva 3.0T Quasar Dual; Philips Electronics, Amsterdam, the Netherlands). Participants were in the supine position with the body‐array coil positioned at the upper abdominal region. Single‐voxel spectra were acquired with the point‐resolved spectroscopy sequence. Intracellular lipid content in skeletal muscle (intramyocellular lipid [IMCL]) and hepatic fat content (HFC) were determined by 1H‐MRS, and the areas of visceral fat tissue (VF) and subcutaneous fat tissue (SCF) were determined by MRI, as described previously27, 28. PFC was measured within a volume of 15 × 15 × 15 mm3 located in the head of the pancreas, avoiding visceral fat and vessels, with the use of 1H‐MRS. The measurement was carried out while the participant had stopped breathing. With this system, simulated lipid and water spectra are detected at 1.4 and 4.8 ppm, respectively (Figure 1). PFC was calculated as the ratio of the area under the lipid peak to the sum of the areas under the lipid peak and the water peak (internal standard), as previously described29, with the use of Scion image analysis software (Scion Corporation, http://www.scioncorp.com). PFC measurement was carried out twice in 20 participants, with the coefficient of variation between the two repeated measurements being 7.4%.
Figure 1.
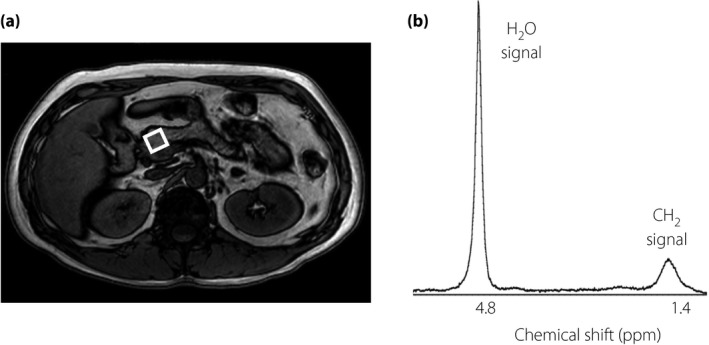
1H magnetic resonance spectroscopy for analysis of pancreatic fat content. (a) Representative magnetic resonance imaging of the pancreas. The square indicates the measured area at the head of the pancreas. (b) Representative 1H magnetic resonance spectroscopy for analysis of pancreatic fat content. The peak of the CH 2 signal is considered as the lipid peak.
Statistical analysis
Data are presented as mean ± standard deviation. The relationships between PFC and other clinical parameters were assessed by univariate correlation and multivariate linear regression analysis with the use of SPSS version 11.0 for Windows software (SPSS, Chicago, IL, USA). We coded the sex variable as men = 0 and women = 1. A P‐value of <0.05 was considered statistically significant.
Results
The study participants included 16 men and 14 women with an age and BMI of 30.8 ± 6.5 years and 20.5 ± 2.0 kg/m2, respectively (Table 1). The results of comprehensive fat content analysis are shown in Table 2, and the relationships between PFC, HFC, IMCL, VF or SCF and clinical or OGTT‐derived parameters are shown in Table 3. PFC was correlated with age, BMI, sex, fasting serum insulin level and serum triglyceride concentration. It was also correlated with indexes of insulin sensitivity‐resistance including HOMA‐IR and the composite index, but not with those of insulin secretion including HOMA‐β, the insulinogenic index and AUCins/glu120. In addition, PFC was correlated with oral DI, an index of insulin secretion adjusted for insulin sensitivity. HFC and VF were correlated with similar sets of parameters, as was PFC, with HFC showing significant correlations with age, BMI, sex, HOMA‐IR, the composite index and oral DI, and VF with age, BMI, sex, fasting serum insulin level, serum triglyceride level, HOMA‐IR and the composite index. IMCL was not correlated with any of the measured parameters, whereas it was previously shown to be correlated with insulin resistance27. SCF was correlated with BMI, AUCins120 and AUCins/glu120.
Table 1.
Demographics, serum lipid concentrations and oral glucose tolerance test‐based parameters for the study participants
| Age (years) | 30.8 ± 6.5 |
| BMI (kg/m2) | 20.5 ± 2.0 |
| Sex (men/women) | 16/14 |
| FPG (mg/dL) | 86.5 ± 6.8 |
| F‐IRI (μU/mL) | 6.5 ± 2.3 |
| Serum TG (mg/dL) | 59.8 ± 21.2 |
| Serum FFAs (mEq/L) | 0.51 ± 0.25 |
| HOMA‐IR | 1.40 ± 0.58 |
| HOMA‐β | 104.5 ± 52.2 |
| Insulinogenic index | 1.20 ± 1.08 |
| Composite index | 6.99 ± 2.60 |
| AUCins120 (min mg/dL) | 4882 ± 1935 |
| AUCins/glu120 | 0.34 ± 0.13 |
| Oral DI | 2.15 ± 0.52 |
Data are mean ± standard deviation. AUCglu120, area under the glucose concentration curve; AUCins120, area under the insulin concentration curve; BMI, body mass index; DI, disposition index; FFAs, free fatty acids; F‐IRI, fasting serum insulin concentration; FPG, fasting plasma glucose concentration; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; TG, triglyceride.
Table 2.
Parameters of comprehensive fat content analysis for the study participants
| PFC (%) | 6.36 ± 3.49 |
| HFC (%) | 4.68 ± 3.48 |
| VF (cm2) | 47.6 ± 24.3 |
| SCF (cm2) | 112.5 ± 44.9 |
| IMCL (AU/Cr) | 9.18 ± 4.39 |
Data are mean ± standard deviation. AU/Cr, arbitrary unit per creatinine; HFC, hepatic fat content; IMCL, intramyocellular lipid content; PFC, pancreatic fat content; SCF, subcutaneous fat area; VF, visceral fat area.
Table 3.
Relationships between tissue fat content and clinical or oral glucose tolerance test‐based parameters
| PFC | HFC | IMCL | VF | SCF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Age | 0.590 | 0.001 | 0.399 | 0.029 | 0.014 | 0.941 | 0.613 | <0.001 | 0.030 | 0.873 |
| BMI | 0.501 | 0.005 | 0.527 | 0.003 | 0.191 | 0.311 | 0.606 | <0.001 | 0.549 | 0.002 |
| Sex | −0.392 | 0.032 | −0.579 | 0.001 | 0.026 | 0.892 | −0.484 | 0.007 | 0.037 | 0.846 |
| FPG | 0.489 | 0.006 | 0.466 | 0.009 | −0.036 | 0.850 | 0.298 | 0.110 | −0.017 | 0.929 |
| F‐IRI | 0.520 | 0.003 | 0.361 | 0.050 | 0.050 | 0.792 | 0.481 | 0.007 | 0.223 | 0.237 |
| Serum TG | 0.439 | 0.015 | 0.345 | 0.062 | −0.066 | 0.730 | 0.523 | 0.003 | 0.204 | 0.279 |
| Serum FFAs | −0.036 | 0.849 | −0.076 | 0.690 | 0.153 | 0.419 | −0.085 | 0.654 | −0.106 | 0.578 |
| HOMA‐β | 0.074 | 0.696 | 0.076 | 0.690 | 0.073 | 0.700 | 0.231 | 0.220 | 0.167 | 0.378 |
| Insulinogenic index | 0.145 | 0.446 | −0.123 | 0.517 | −0.192 | 0.308 | 0.037 | 0.845 | 0.068 | 0.721 |
| AUCins120 | 0.332 | 0.073 | 0.342 | 0.064 | 0.033 | 0.862 | 0.337 | 0.068 | 0.396 | 0.030 |
| AUCins/glu120 | 0.203 | 0.282 | 0.173 | 0.360 | 0.036 | 0.850 | 0.200 | 0.290 | 0.397 | 0.030 |
| HOMA‐IR | 0.619 | <0.001 | 0.483 | 0.007 | 0.034 | 0.858 | 0.558 | 0.001 | 0.282 | 0.131 |
| Composite index | −0.433 | 0.017 | −0.416 | 0.022 | −0.059 | 0.755 | −0.388 | 0.034 | −0.197 | 0.296 |
| Oral DI | −0.403 | 0.027 | −0.435 | 0.016 | 0.018 | 0.925 | −0.347 | 0.060 | 0.184 | 0.330 |
AUCglu120, area under the glucose concentration curve; AUCins120, area under the insulin concentration curve; BMI, body mass index; DI, disposition index; FFAs, free fatty acids; F‐IRI, fasting serum insulin concentration; FPG, fasting plasma glucose concentration; HFC, hepatic fat content; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; IMCL, intramyocellular lipid content; PFC, pancreatic fat content; SCF, subcutaneous fat area; TG, triglyceride; VF, visceral fat area.
Given that several measures of fat content including PFC, HFC, and VF were correlated with age, BMI and sex, we next analyzed the relationships between fat content measures and indexes of insulin secretion and sensitivity‐resistance after adjustment for age, BMI, and sex (Table 4). After this adjustment, PFC was correlated with only HOMA‐IR, VF was correlated with HOMA‐β and HOMA‐IR, and SCF was correlated with AUCins120 and AUCins/glu120, whereas HFC and IMCL did not show any significant correlations.
Table 4.
Relationships between tissue fat content and indexes of insulin secretion or insulin sensitivity‐resistance after adjustment for age, body mass index and sex
| PFC | HFC | IMCL | VF | SCF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| HOMA‐β | 0.151 | 0.453 | 0.097 | 0.630 | 0.079 | 0.696 | 0.406 | 0.036 | 0.226 | 0.258 |
| Insulinogenic index | 0.219 | 0.273 | −0.123 | 0.542 | −0.157 | 0.435 | 0.104 | 0.607 | 0.259 | 0.193 |
| AUCins120 | 0.276 | 0.164 | 0.189 | 0.346 | 0.027 | 0.893 | 0.260 | 0.190 | 0.506 | 0.007 |
| AUCins/glu120 | 0.241 | 0.227 | 0.096 | 0.635 | 0.040 | 0.843 | 0.231 | 0.246 | 0.532 | 0.004 |
| HOMA‐IR | 0.498 | 0.008 | 0.294 | 0.137 | 0.014 | 0.946 | 0.394 | 0.042 | 0.313 | 0.111 |
| Composite index | −0.337 | 0.086 | −0.218 | 0.275 | −0.057 | 0.776 | −0.232 | 0.244 | −0.230 | 0.248 |
| Oral DI | −0.177 | 0.378 | −0.253 | 0.203 | 0.032 | 0.874 | −0.050 | 0.804 | 0.318 | 0.106 |
AUCglu120, area under the glucose concentration curve; AUCins120, area under the insulin concentration curve; DI, disposition index; HFC, hepatic fat content; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; IMCL, intramyocellular lipid content; PFC, pancreatic fat content; SCF, subcutaneous fat area; VF, visceral fat area.
We finally analyzed the relationships between PFC and other fat content measures and BMI. PFC was significantly correlated with all these parameters with the exception of IMCL (Figure 2). The strongest correlation was observed with HFC, and the second strongest with VF.
Figure 2.
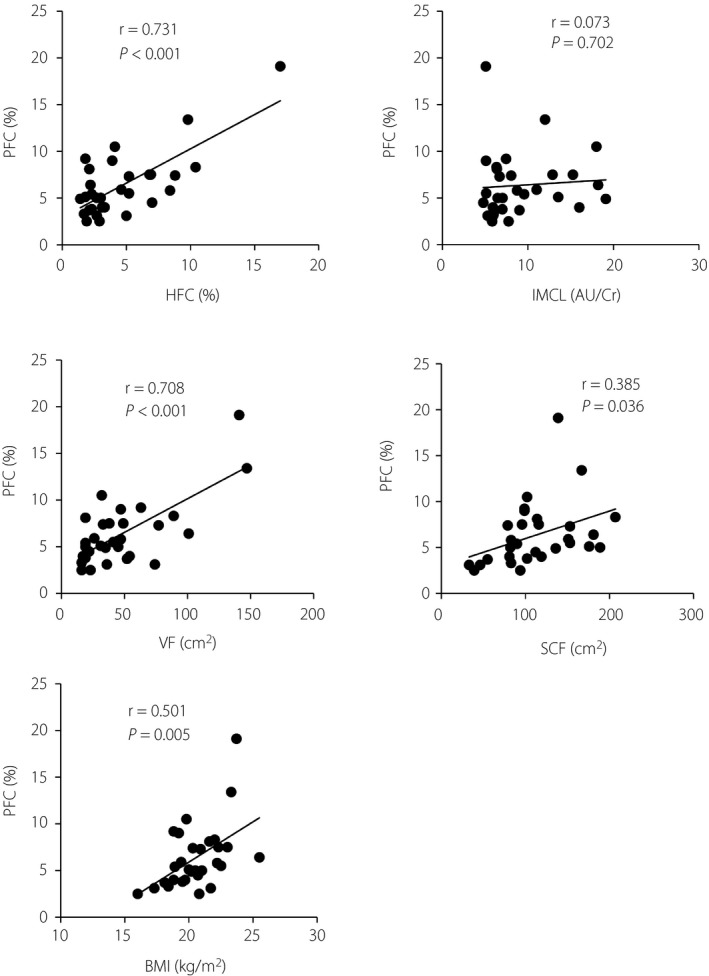
Relationships between pancreatic fat content (PFC) and other parameters of fat analysis and body mass index (BMI) for the study participants. AU/Cr, arbitrary unit per creatinine; HFC, hepatic fat content; IMCL, intramyocellular lipid; SCF, subcutaneous fat tissue.
Discussion
As far as we are aware, the present study is the first to evaluate PFC by 1H‐MRS in Japanese individuals. We found that PFC was correlated with indexes of insulin sensitivity‐resistance (HOMA‐IR and the composite index), but not with those of insulin secretion (HOMA‐β, the insulinogenic index or AUCins/glu120). A correlation between PFC and insulin sensitivity‐resistance was previously described for overweight or obese non‐Asian adults and children30, 31. We have now shown that this relationship is conserved in Japanese individuals with normal body mass (BMI of 20.5 ± 2.0 kg/m2). The correlation of PFC with HOMA‐IR remained significant after adjustment for age, BMI and sex, showing that the relationship between PFC and insulin resistance is independent of these factors.
A previous study has shown that HFC and were correlated with indexes of insulin sensitivity‐resistance21. In the present study, although HF was correlated with both HOMA‐IR and the composite index, these correlations did not remain significant after the adjustment of age, BMI and sex, suggesting that HF is heavily influenced by these parameters. IMCL of the current study participants was relatively low and distributed in a narrow range, which might explain the reason why the correlation of IMCL with insulin sensitivity‐resistance was not observed.
Previous studies have yielded discrepant results regarding the relationship between PFC and the capacity for insulin secretion8, 9, 10, 11, 12, 13. Although the reason for this discordance is unclear, one study found that PFC was correlated with parameters of insulin secretion in Caucasians, but not in Hispanics, with NGT10. Furthermore, another study showed that the reduction in PFC after bariatric surgery was correlated with the improvement in insulin secretion in individuals with diabetes, but not in those with NGT32. It is thus possible that the influence of pancreatic fat on insulin secretion is dependent on various factors including ethnicity and the level of glucose tolerance. A previous study has shown that insulin secretory response of East Asians is lower than those of Caucasians or Africans33. Such physiological characteristics of East Asian ethnicity might have influenced the results of the present study. CT values of the pancreas were shown to be correlated with the insulinogenic index in Japanese individuals undergoing a health checkup, including individuals with NGT, impaired glucose tolerance or diabetes mellitus21. The relationship between 1H‐MRS‐based PFC and the capacity for insulin secretion thus warrants investigation in individuals other than those with NGT.
We found that PFC was highly correlated with HFC and VF. Similar observations were described for individuals with several pathological conditions, including non‐alcoholic fatty liver disease and pediatric obesity29, 30, 31. While VF is widely accepted as a contributing factor to insulin resistance34, 35, whether HFC is a cause or result of insulin resistance remains unclear36, 37. Although the nature of the causal relationship between PFC and insulin resistance is currently unknown, it is possible that PFC is a result of insulin resistance. The exaggerated accumulation of VF is associated with chronic inflammation in this tissue and with the alterations in the secretion of adipokines, as well as in the metabolism of fatty acids, which in turn contribute to the development of insulin resistance37. It is thus possible that one of these factors is also related to the accumulation of fat in the pancreas. Given that fat accumulates only in parenchymal cells in the liver, HFC is upregulated by an increase in the influx or synthesis of fatty acids in, or by a decrease in the secretion of lipoproteins from, hepatocytes38. Fat accumulation in the pancreas, in contrast, reflects the infiltration of adipocytes in interstitial tissue, replacement of parenchymal cells with fat and the accumulation of fat in parenchymal cells4. It remains to be elucidated why the accumulation of fat in these different tissues through apparently different mechanisms occurs concomitantly. Whatever the mechanism, PFC might serve as a clinical marker of insulin resistance as HFC does.
In conclusion, we have shown that insulin resistance, but not insulin secretion, is correlated with PFC in a manner independent of age, sex and BMI in Japanese individuals with NGT. Given that the BMI of the study participants was low and distributed within a narrow range, it is unclear whether the current findings are broadly applicable to the general Japanese population. Further studies of individuals with different backgrounds, such as those with different body compositions or different levels of glucose tolerance, are warranted to shed light on the physiological relevance of PFC.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
We thank Naoko Hashimoto, Tomokazu Matsuda and Michinori Takabe for assistance with data collection, as well as Takeshi Ohara for helpful discussion. This study was supported by a Research Grant from the Japan Diabetes Foundation to YH.
J Diabetes Investig 2018;9: 505–511
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000003753
References
- 1. Krssak M, Falk Petersen K, Dresner A, et al Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999; 42: 113–116. [DOI] [PubMed] [Google Scholar]
- 2. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 3. Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 2008; 295: E1009–E1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol 2011; 8: 169–177. [DOI] [PubMed] [Google Scholar]
- 5. Thomas EL, Fitzpatrick JA, Malik SJ, et al Whole body fat: content and distribution. Prog Nucl Magn Reson Spectrosc 2013; 73: 56–80. [DOI] [PubMed] [Google Scholar]
- 6. Lee Y, Hirose H, Ohneda M, et al Beta‐cell lipotoxicity in the pathogenesis of non‐insulin‐dependent diabetes mellitus of obese rats: impairment in adipocyte‐beta‐cell relationships. Proc Natl Acad Sci USA 1994; 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giacca A, Xiao C, Oprescu AI, et al Lipid‐induced pancreatic β‐cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 2011; 300: E255–E262. [DOI] [PubMed] [Google Scholar]
- 8. Tushuizen ME, Bunck MC, Pouwels PJ, et al Pancreatic fat content and beta‐cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916–2921. [DOI] [PubMed] [Google Scholar]
- 9. Heni M, Machann J, Staiger H, et al Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev 2010; 26: 200–205. [DOI] [PubMed] [Google Scholar]
- 10. Szczepaniak LS, Victor RG, Mathur R, et al Pancreatic steatosis and its relationship to β‐cell dysfunction in humans: racial and ethnic variations. Diabetes Care 2012; 35: 2377–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saisho Y, Butler AE, Meier JJ, et al Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type‐2 diabetes. Clin Anat 2007; 20: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Zijl NJ, Goossens GH, Moors CC, et al Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β‐cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011; 96: 459–467. [DOI] [PubMed] [Google Scholar]
- 13. Begovatz P, Koliaki C, Weber K, et al Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia 2015; 58: 1646–1655. [DOI] [PubMed] [Google Scholar]
- 14. Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas 2010; 65: 315–319. [DOI] [PubMed] [Google Scholar]
- 15. NCD Risk Factor Collaboration . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 17. Seto WK, Yuen MF. Nonalcoholic fatty liver disease in Asia: emerging perspectives. J Gastroenterol 2017; 52: 164–174. [DOI] [PubMed] [Google Scholar]
- 18. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity . New criteria for ‘obesity disease, Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 19. WHO . Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Consultation. WHO Technical Report Series Number 854. Geneva: World Health Organization, 1995. [PubMed] [Google Scholar]
- 20. Apovian CM, Aronne LJ. The 2013 American Heart Association/American College of Cardiology/The Obesity Society Guideline for the Management of Overweight and Obesity in Adults: What Is New About Diet, Drugs, and Surgery for Obesity? Circulation 2015; 132: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 21. Yokota K, Fukushima M, Takahashi Y, et al Insulin secretion and computed tomography values of the pancreas in the early stage of the development of diabetes. J Diabetes Investig 2012; 3: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamazaki H, Tsuboya T, Katanuma A, et al Lack of independent association between fatty pancreas and incidence of type 2 diabetes: 5‐year Japanese cohort study. Diabetes Care 2016; 39: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 23. Lingvay I, Esser V, Legendre JL, et al Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab 2009; 94: 4070–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee Y, Lingvay I, Szczepaniak LS, et al Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond) 2010; 34: 396–400. [DOI] [PubMed] [Google Scholar]
- 25. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 26. Komada H, Sakaguchi K, Takeda K, et al Age‐dependent decline in β‐cell function assessed by an oral glucose tolerance test‐based disposition index. J Diabetes Investig 2011; 2: 293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maeda K, Ishihara K, Miyake K, et al Inverse correlation between serum adiponectin concentration and hepatic lipid content in Japanese with type 2 diabetes. Metabolism 2005; 54: 775–780. [DOI] [PubMed] [Google Scholar]
- 28. Teranishi T, Ohara T, Maeda K, et al Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism 2007; 56: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 29. Hu HH, Kim HW, Nayak KS, et al Comparison of fat‐water MRI and single‐voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity 2010; 18: 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong VW, Wong GL, Yeung DK, et al Fatty pancreas, insulin resistance, and β‐cell function: a population study using fat‐water magnetic resonance imaging. Am J Gastroenterol 2014; 109: 589–597. [DOI] [PubMed] [Google Scholar]
- 31. Pacifico L, Di Martino M, Anania C, et al Pancreatic fat and β‐cell function in overweight/obese children with nonalcoholic fatty liver disease. World J Gastroenterol 2015; 21: 4688–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steven S, Hollingsworth KG, Small PK, et al Weight loss decreases excess pancreatic triacylglycerol specifically in type 2 diabetes. Diabetes Care 2016; 39: 158–165. [DOI] [PubMed] [Google Scholar]
- 33. Kodama K, Tojjar D, Yamada S, et al Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta‐analysis. Diabetes Care 2013; 36: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuzawa Y, Shimomura I, Nakamura T, et al Pathophysiology and pathogenesis of visceral fat obesity. Ann N Y Acad Sci 1995; 748: 399–406. [DOI] [PubMed] [Google Scholar]
- 35. Lopes HF, Corrêa‐Giannella ML, Consolim‐Colombo FM, et al Visceral adiposity syndrome. Diabetol Metab Syndr 2016; 8: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petersen KF, Dufour S, Befroy D, et al Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005; 54: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: is insulin resistance the link? Mol Cell Endocrinol 2015; 418: 55–65. [DOI] [PubMed] [Google Scholar]
- 38. Berlanga A, Guiu‐Jurado E, Porras JA, et al Molecular pathways in non‐alcoholic fatty liver disease. Clin Exp Gastroenterol 2014; 7: 221–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


