Abstract
Purpose/Objectives
To examine differences in opportunity and eligibility for cancer clinical trial (CCT) participation based on sociodemographic and disease characteristics.
Design
A matched cross-sectional study including a prospective oral questionnaire and retrospective electronic medical record (EMR) review.
Setting
A single hospital in a large academic National Cancer Institute–designated cancer center in Philadelphia, Pennsylvania.
Sample
44 Black or Hispanic and 44 Non-Hispanic White newly diagnosed individuals matched on cancer type and age (plus or minus five years).
Methods
Participants answered a questionnaire to capture self-reported opportunity for CCT participation, sociodemographic information, and cancer type. With consent, the authors completed a retrospective review of the EMR to assess eligibility and collect cancer stage and performance status.
Main Research Variables
Opportunity and eligibility for CCT participation.
Findings
Most participants (78%) had no opportunity for participation and were ineligible for all available trials. No differences were noted in opportunity for participation or eligibility based on race or ethnicity. Participants with late-stage disease were more likely to have opportunity and be eligible for CCT participation (p = 0.001). Those with private insurance were less likely to have opportunity for participation (p = 0.05).
Conclusions
Limited trial availability and ineligibility negatively influenced opportunity for CCT participation for all populations. Levels of under-representation for CCT participation likely vary within and across sociodemographic and disease characteristics, as well as across healthcare settings.
Implications for Nursing
The unique roles of nurse navigators and advanced practice nurses can be leveraged to increase opportunities for CCT participation for all populations.
Keywords: cancer clinical trial, opportunity, eligibility, under-represented groups
Racial and ethnic minority populations, older adults, and the economically disadvantaged are significantly under-represented as cancer clinical trial (CCT) participants (Murthy, Krumholz, & Gross, 2004; Sateren et al., 2002). Inequitable participation in CCTs decreases the generalizability of results and diminishes the chance for under-represented groups to receive new and potentially life-saving treatments. A large portion of literature on under-representation identifies patient attitudes as a barrier to CCT participation, but evidence challenges this view (Comis, Miller, Aldige, Krebs, & Stoval, 2003; Langford et al., 2014; Markman, Petersen, & Montgomery, 2008; Mohd Noor et al., 2013; Wallington et al., 2012; Wendler et al., 2006). Research suggests that under-represented groups are just as willing to participate in clinical trials as well-represented groups but receive fewer opportunities for participation (Wendler et al., 2006). Opportunity for participation is defined as an offer for screening and/or enrollment in a CCT from a healthcare provider or researcher. An important determinant of opportunity for participation is eligibility (Ford et al., 2005, 2008), which can be defined as the key attributes or characteristics a person must have to participate in a CCT.
Available evidence suggests that sociodemographic factors, particularly race, ethnicity, age, language, insurance, and socioeconomic status (SES), are associated with decreased opportunity and eligibility for CCT participation among under-represented groups (Byrne, Tannenbaum, Gluck, Hurley, & Antoni, 2014; Javid et al., 2012; Klamerus et al., 2010; Mohd Noor et al., 2013; Penberthy et al., 2012). Investigators also demonstrated that eligibility can be negatively influenced by disease characteristics, including cancer type, advanced stage, and poor performance status (Baggstrom et al., 2011; Gonzalez et al., 2013; Penberthy et al., 2012). Data on eligibility are reinforced by data on disease characteristics for under-represented groups, who often are medically underserved and experience a disproportionate burden of cancer incidence, late-stage disease, and comorbidities (Lantz et al., 2006; National Cancer Institute [NCI], 2008; Virnig, Baxter, Habermann, Feldman, & Bradley, 2009). These characteristics may lead to disproportionate ineligibility among these groups and, in turn, the potential for decreased opportunity for participation.
Understanding opportunity and eligibility to participate in CCTs is relevant to nursing practice because of nurses’ varied and important roles as investigators, healthcare providers, clinical researchers, and patient navigators. Information is lacking on the number of potentially eligible patients who may not receive opportunity for participation. This information will assist nurses in developing and testing interventions to improve opportunity for participation for diverse populations and ensure the generalizability of CCT findings. Therefore, the aims of this study were to (a) examine the differences in sociodemographic variables (age, race and ethnicity, sex, language, insurance status, education, income) and disease characteristics (cancer type, stage, performance status) among patients with opportunity and without opportunity for CCT participation; (b) examine the differences in sociodemographic variables and disease characteristics among patients eligible and ineligible for CCT participation; and (c) examine the differences in sociodemographic variables and disease characteristics among the following groups: opportunity-eligible, opportunity-ineligible, no opportunity-eligible, or no opportunity-ineligible.
Methods
The authors used a cross-sectional matched design with a prospective, oral patient questionnaire delivered by the research team, followed by a retrospective electronic medical record (EMR) review, to explore opportunity and eligibility for CCT participation. The study was conducted in the outpatient medical oncology clinics of a single hospital in an NCI–designated comprehensive cancer center in Philadelphia, Pennsylvania.
Theoretical Framework
This work was guided by the theoretical model developed by Ford et al. (2005, 2008) to examine barriers or promoters of CCT participation for under-represented groups. The theory posits that, for a person to accept or refuse participation in a clinical trial, he or she must be aware that the trial exists and have the opportunity to participate (Ford et al., 2008). Barriers or promoters exist for awareness, opportunity, and acceptance or refusal that are moderated by sociodemographic variables. Eligibility is a potential barrier or promoter to opportunity for participation identified within the model (Ford et al., 2008). The framework provides a balanced explanation of barriers or promoters that can be used to develop interventions to increase participation.
Sample
Following University of Pennsylvania institutional review board and scientific review committee approval, the authors obtained administrative data identifying all Black or Hispanic patients with leukemia or breast, lung, or kidney cancer who had a new patient visit with a medical oncologist in the oncology outpatient clinics of a single hospital from January to June 2013. This population included newly diagnosed patients and those with existing cancer. The authors matched Black and Hispanic patients with all possible Non-Hispanic White patients seen during the same time period who were of similar age (plus or minus five years) with the same cancer type. A random number was assigned to all of the Non-Hispanic White matches for each Black and Hispanic study participant. For each Black or Hispanic new patient who chose to participate in the study, the authors contacted the Non-Hispanic White match for participation with the lowest random number to create a 1:1 match ratio.
Participants included in this study (a) were aged 21 years or older, (b) self-identified as Black, Hispanic, or Non-Hispanic White, (c) were at least four weeks post-completion of a new patient visit with a medical oncologist that occurred from January to June 2013, and (d) were diagnosed with a cancer that had a large number of accruing phase I–III treatment trials supported by the cancer center’s centralized clinical research unit (CRU), including leukemia and breast, lung, and kidney cancer. The authors chose cancer types with high numbers of accruing treatment trials to maximize the number of participants eligible for CCTs. Patients who could not speak or understand spoken English or Spanish and those who were not competent to provide informed consent were excluded.
Procedures
The authors stratified participants into groups by opportunity status (opportunity or no opportunity) and eligibility status (eligible for enrolling CCTs or ineligible for enrolling CCTs) using a brief patient questionnaire and, with verbal consent from the participant, a retrospective EMR review. The authors contacted new patients via telephone or in person to complete the questionnaire. Sociodemographic information and cancer type were captured, and participants were asked to self-report opportunity—specifically whether or not they were offered screening or enrollment in any therapeutic CCTs by their healthcare providers or a researcher. To detect recall bias, the authors confirmed responses regarding whether a participant had opportunity for CCT participation using (a) documentation of an offer for trial screening and/or enrollment by a healthcare provider in the EMR, and/or (b) documentation of screening and/or enrollment in the CRU’s clinical trials database.
With the verbal informed consent of the patient, the authors performed a retrospective EMR review to collect information about cancer stage and performance status, as well as to stratify patients by eligibility status. Patients who provided permission for EMR review were systematically screened for all accruing therapeutic phase I–III CCTs for their cancer site using protocol eligibility criteria and information documented in the medical record. The authors obtained a list of accruing CCTs for each cancer type, as well as complete eligibility criteria, from the cancer center’s CRU. To account for any amendments to the eligibility criteria made during the time period of interest, the authors obtained all versions of eligibility criteria for each protocol with corresponding dates of use based on the dates the revised criteria were approved by the institutional review board and scientific review committee.
Opportunity patients were defined as those who indicated in the questionnaire that they were offered screening and/or enrollment in a therapeutic CCT. No-opportunity patients were defined as those who indicated in the questionnaire that they were not offered screening and/or enrollment in a therapeutic CCT. Eligible patients were defined as those who met all evaluable eligibility criteria (meaning that enough information was available to assess whether a criterion is met) for at least one open and enrolling phase I–III therapeutic CCT. Ineligible patients were defined as those who did not meet all evaluable criteria for any open and enrolling phase I–III therapeutic CCTs. The authors stratified participants based on these definitions into opportunity–no opportunity (O/N) groups and eligible–ineligible (E/I) groups. In addition, the authors stratified participants into the following four groups for additional analyses to determine whether eligible patients were receiving opportunity for participation: opportunity-eligible (O/E), no opportunity-eligible (N/E), opportunity-ineligible (O/I), and no opportunity-ineligible (N/I).
Data Analysis Plan
Contingency tables were constructed and differences in categorical sociodemographic variables and disease characteristics were examined among O/N and E/I patients using two-tailed Fisher’s exact tests. The authors then examined differences in age between the groups using independent sample two-tailed t tests. Similarly, descriptive contingency tables were constructed and differences in categorical sociodemographic variables and disease characteristics were examined among the four groups (O/E, N/E, O/I, and N/I) using a series of two-sided Fisher’s exact tests. The authors examined differences in age among the four groups using a one-way analysis of variance. All analyses were performed using STATA®, version 13.1.
Results
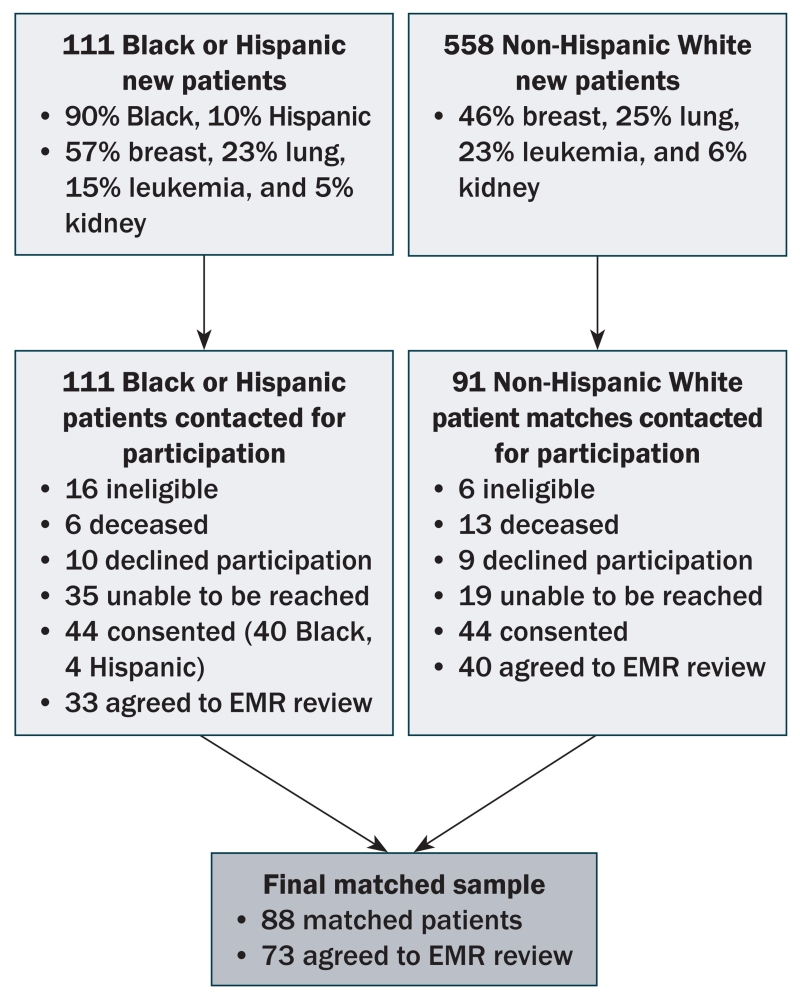
The final sample included 88 participants (44 Black or Hispanic, 44 Non-Hispanic White) matched in a 1:1 ratio based on cancer type and age (plus or minus five years) (see Figure 1). Characteristics of the matched sample are located in Table 1. The majority of participants were women (86%) with breast cancer (65%). Forty-nine percent of the sample had early-stage disease. Non-Hispanic White participants were more likely than Black or Hispanic participants to have private insurance (84% versus 52%, p = 0.002), have a graduate level education (32% versus 9%, p = 0.02), and make more than $100,000 per year before taxes (59% versus 6%, p = 0.00). The groups were no different in terms of other sociodemographic and disease characteristics.
FIGURE 1. Sample Selection.
EMR—electronic medical record
TABLE 1. Sociodemographic and Disease Characteristics by Matched Groups (Black or Hispanic Versus Non-Hispanic White).
| Black or Hispanica (N = 44) |
Non-Hispanic White (N = 44) |
||||
|---|---|---|---|---|---|
| Characteristic | SD | SD | pb | ||
| Age (years) | 56.3 | 12.5 | 56 | 11.5 | 0.93 |
| Characteristic | n | n | pb |
|---|---|---|---|
| Sex | 0.76 | ||
| Female | 39 | 37 | |
| Male | 5 | 7 | |
| Insurance c | 0.002 | ||
| Private | 22 | 37 | |
| Medicare | 15 | 7 | |
| Medicaid | 5 | – | |
| Education | 0.02 | ||
| High school or less | 28 | 18 | |
| Associates or bachelor’s | 11 | 12 | |
| Graduate or professional | 4 | 14 | |
| Annual household income ($) | 0.00 | ||
| Less than 25,000 | 12 | 1 | |
| 25,000–49,000 | 13 | 5 | |
| 50,000–99,000 | 9 | 8 | |
| 100,000 or greater | 2 | 20 | |
| Language | 0.36 | ||
| English | 40 | 43 | |
| Bilingual | 3 | 1 | |
| Cancer type | 1 | ||
| Breast | 29 | 28 | |
| Lung | 8 | 7 | |
| Leukemia | 5 | 6 | |
| Other | 2 | 3 | |
| Cancer staged | 0.97 | ||
| 0–II | 16 | 20 | |
| III–IV | 8 | 11 | |
| Recurrent or relapsed | 5 | 4 | |
| Other | 4 | 5 | |
| Performance statusd | 0.43 | ||
| ECOG 0 | 14 | 16 | |
| ECOG 1 | 4 | 1 | |
| ECOG 2 | 2 | 1 |
Black or Hispanic and Non-Hispanic White participants matched in a 1:1 ratio based on cancer type and age (plus or minus five years).
All p values are two-sided Fisher’s exact tests performed on non-missing observations.
One participant with no insurance was removed from this analysis.
This information was only collected if available for participants who consented to electronic medical record review (n = 73).
ECOG—Eastern Cooperative Oncology Group
Note.Unless otherwise indicated, missing observations reflect participant non-response or that information was not present in the electronic medical record.
Descriptive information about the number and type of CCTs open and enrolling from January to June 2013 is presented in Table 2. Fifty-four phase I–III treatment CCTs were supported by the cancer center’s CRU accruing at some point during the six-month study period. Patients with lung cancer and leukemia had the most open and enrolling trials available. Accruing studies were predominantly phase II (32%) or III (30%), sponsored by pharmaceutical companies (54%), and designed for patients with advanced, relapsed, or refractory disease (72%).
TABLE 2. Therapeutic Cancer Clinical Trials Open and Enrolling During the Study Period (N = 54)a.
| Trial Characteristic | n |
|---|---|
| Cancer type | |
| Leukemia | 18 |
| Lung | 14 |
| Breast | 8 |
| Kidney | 8 |
| Multiple | 6 |
| • Lung and kidney | 1 |
| • Breast, lung, and kidney | 5 |
| Cancer stage | |
| Early or untreated | 10 |
| Advanced, relapsed, or refractory | 39 |
| Both | 5 |
| Phase | |
| I | 10 |
| I/II | 9 |
| II | 17 |
| III | 16 |
| Other | 2 |
| Sponsor type | |
| Pharmaceutical | 29 |
| Cooperative group | 14 |
| Institution (investigator-initiated) | 11 |
Studies were open and enrolling for some or all of the six-month study period.
Opportunity Versus No-Opportunity Groups
Differences in self-reported opportunity (opportunity versus no opportunity) for CCT participation based on sociodemographic and disease characteristics are presented in Table 3. Most participants (79%) reported that they did not have opportunity for CCT participation. Those who reported having no opportunity were more likely to have private insurance (74%) as opposed to Medicare (22%) or Medicaid (4%) (p = 0.05). Patients who reported having opportunity for CCT participation were more likely to have stage III or IV disease (67%) compared to stages 0–II (8%), recurrent or relapsed (8%), or other (i.e., refractory, remission, multiple cancers, or unknown stage) (17%) (p = 0.001). No statistically significant differences were noted between participants with and without opportunity for CCT participation based on race or ethnicity, age, gender, education, annual household income, primary spoken language, cancer type, or performance status. The authors were able to confirm the participant’s self-reported opportunity with the EMR in 96% of cases and in the CRU’s electronic clinical trials database in 90% of cases.
TABLE 3. Comparison of Opportunity Versus No Opportunity (N = 87)a and Eligible Versus Ineligible (N = 73)b Patients by Sociodemographic and Disease Characteristics.
| Opportunity (n = 18) |
No Opportunity (n = 69) |
Eligible (n = 12) |
Ineligible (n = 61) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | SD | SD | pc | SD | SD | pc | ||||
| Age (years) | 59.6 | 8.9 | 55.2 | 12.5 | 0.17 | 55.4 | 9.5 | 55.3 | 11.5 | 0.98 |
| Characteristic | n | n | pc | n | n | pc |
|---|---|---|---|---|---|---|
| Race or ethnicity | 0.43 | 0.53 | ||||
| Black or Hispanic | 11 | 33 | 4 | 29 | ||
| Non-Hispanic White | 7 | 36 | 8 | 32 | ||
| Sex | 0.69 | 0.08 | ||||
| Female | 15 | 61 | 8 | 54 | ||
| Male | 3 | 8 | 4 | 7 | ||
| Insuranced | 0.05 | 1 | ||||
| Private | 8 | 50 | 8 | 42 | ||
| Medicare | 7 | 15 | 3 | 14 | ||
| Medicaid | 2 | 3 | 1 | 4 | ||
| Education | 0.09 | 0.26 | ||||
| High school or less | 13 | 32 | 9 | 29 | ||
| Associate or bachelor’s | 3 | 20 | 2 | 17 | ||
| Graduate or professional | 1 | 17 | 1 | 15 | ||
| Annual household income ($) | 0.28 | 0.66 | ||||
| Less than 25,000 | 2 | 11 | 2 | 8 | ||
| 25,000–49,999 | 6 | 12 | 4 | 12 | ||
| 50,000–99,999 | 2 | 15 | 1 | 13 | ||
| 100,000 or greater | 2 | 19 | 3 | 17 | ||
| Language | 1 | 0.42 | ||||
| English | 16 | 66 | 11 | 59 | ||
| Bilingual | 1 | 3 | 1 | 2 | ||
| Cancer type | 0.08 | 0.13 | ||||
| Breast | 8 | 49 | 5 | 41 | ||
| Lung | 4 | 11 | 4 | 9 | ||
| Leukemia | 5 | 6 | 3 | 7 | ||
| Other | 1 | 3 | – | 4 | ||
| Cancer stagee | 0.001 | 0.00 | ||||
| 0–II | 1 | 35 | – | 36 | ||
| III–IV | 8 | 10 | 9 | 10 | ||
| Recurrent or relapsed | 1 | 8 | – | 9 | ||
| Other | 2 | 7 | 3 | 6 | ||
| Performance statuse | 0.41 | 1 | ||||
| ECOG 0 | 9 | 21 | 7 | 23 | ||
| ECOG 1 | – | 5 | 1 | 4 | ||
| ECOG 2 | 1 | 2 | – | 3 |
One participant unsure about opportunity status removed from sample.
Fifteen participants declined electronic medical record review.
All p values are two-sided Fisher’s exact tests performed on non-missing observations.
One participant with no insurance was removed from this analysis.
This information was only collected if available for participants that consented to electronic medical record review (n = 73).
ECOG—Eastern Cooperative Oncology Group
Note. Unless otherwise indicated, missing observations reflect participant non-response or that information was not present in the electronic medical record.
Eligible Versus Ineligible Groups
Differences in eligibility for CCT participation (eligible versus ineligible) are reported in Table 3. Eligibility status was only assessed for participants who consented to EMR review (n = 73 of 88, 83%). The majority of participants (84%) were ineligible for accruing clinical trials. Ineligible participants were more likely to have stage 0–II disease (59%), as opposed to stage III or IV (16%), recurrent or relapsed disease (15%), or other (10%) (p ≤ 0.001). Those who were eligible for CCT participation were more likely to have stage III or IV disease (75%) compared to stage 0–II (0%), recurrent or relapsed (0%), and others (25%) (p ≤ 0.001). No differences were noted in eligibility based on race or ethnicity, gender, insurance status, education, annual household income, primary spoken language, age, cancer type, or performance status.
Opportunity and Eligibility Groups
Differences in opportunity and eligibility (O/E versus O/I versus N/E versus N/I) are reported in Table 4. Most participants (78%) fell into the N/I group, meaning they had no opportunity for CCT participation and also were ineligible. Of 12 eligible patients, 4 patients fell into the N/E group. Those with stage 0–II cancers were more likely to fall into the N/I group (61%) compared to stage III or IV (14%), those with relapsed or recurrent disease (14%), or others (11%) (p < 0.001). No differences were noted between the four groups based on race or ethnicity, sex, education, insurance status, annual household income, primary spoken language, age, cancer type, and performance status.
TABLE 4. Comparison of Opportunity and Eligibility Groups by Sociodemographic and Disease Characteristics for Participants Who Consented to Electronic Medical Record Review (N = 73)a.
| Opportunity- Eligible Group (n = 8) |
Opportunity- Ineligible Group (n = 4) |
No Opportunity- Eligible Group (n = 4) |
No Opportunity- Ineligible Group (n = 57) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | SD | SD | SD | SD | pb | ||||
| Age (years) | 58.9 | 7.4 | 55.5 | 13.6 | 48.5 | 10.3 | 55.3 | 11.5 | 0.52 |
| Characteristic | n | n | n | n | pb |
|---|---|---|---|---|---|
| Race or ethnicity | 0.23 | ||||
| Black or Hispanic | 4 | 3 | – | 26 | |
| Non-Hispanic White | 4 | 1 | 4 | 31 | |
| Sex | 0.21 | ||||
| Male | 3 | – | 1 | 7 | |
| Female | 5 | 4 | 3 | 50 | |
| Insurancec | 0.13 | ||||
| Private | 4 | 1 | 4 | 41 | |
| Medicare | 3 | 2 | – | 12 | |
| Medicaid | 1 | 1 | – | 3 | |
| Education | 0.67 | ||||
| High school or less | 6 | 3 | 3 | 26 | |
| Associate or bachelor’s | 1 | 1 | 1 | 16 | |
| Graduate or professional | 1 | – | – | 15 | |
| Annual household income ($) | 0.74 | ||||
| Less than 25,000 | 1 | 1 | 1 | 7 | |
| 25,000–49,999 | 3 | 1 | 1 | 11 | |
| 50,000–99,999 | 1 | – | – | 13 | |
| 100,000 or greater | 2 | – | 1 | 17 | |
| Language | 0.53 | ||||
| English | 7 | 4 | 4 | 55 | |
| Bilingual | 1 | – | – | 2 | |
| Cancer type | 0.17 | ||||
| Breast | 2 | 2 | 3 | 39 | |
| Lung | 3 | 1 | 1 | 8 | |
| Leukemia | 3 | 1 | – | 6 | |
| Other | – | – | – | 4 | |
| Cancer staged | 0.00 | ||||
| 0–II | – | 1 | – | 35 | |
| III–IV | 6 | 2 | 3 | 8 | |
| Recurrent or relapsed | – | 1 | – | 8 | |
| Other | 2 | – | 1 | 6 | |
| Performance statusd | 0.15 | ||||
| ECOG 0 | 7 | 2 | – | 21 | |
| ECOG 1 | – | – | 1 | 4 | |
| ECOG 2 | – | 1 | – | 2 |
Fifteen participants declined electronic medical record review.
All p values are two-sided Fisher’s exact tests performed on non-missing observations.
One participant with no insurance was removed from this analysis.
This information was collected if available in the electronic medical record.
ECOG—Eastern Cooperative Oncology Group
Note. Unless otherwise indicated, missing observations reflect participant non-response or that information was not present in the electronic medical record.
Discussion
Benchmarks for Opportunity and Eligibility Rates
The results indicate that opportunity and eligibility for CCT participation among new patients with cancer is low across a variety of sociodemographic populations. Complicating the picture, limited data are available for comparison of rates of opportunity for CCT participation, but other investigators have also found low rates of opportunity. For example, Simon et al. (2004) examined offers for trial participation for 319 newly evaluated patients with breast cancer seen during a one-year period at a large comprehensive cancer center and found that 106 (33%) had opportunity for CCT enrollment. A large comprehensive cancer center reported that 742 of 1,955 patients (38%) seen during a three-year period who had a therapeutic trial evaluation were eligible for CCT participation (Penberthy et al., 2012), whereas 262 of 1,012 new patients (26%) seen at a community cancer center during a one-year period were reported eligible for CCTs (Go et al., 2006).
Given that the current authors specifically included participants who had cancer types with large numbers of accruing trials available to maximize the number of eligible patients, higher rates of opportunity and eligibility were expected. Because comparatively low rates of opportunity and eligibility were found, additional research should focus on determining feasible and appropriate rates of opportunity and eligibility for CCT participation across a variety of healthcare settings over time to explore suitable benchmarks.
Patients Who Lack Opportunity and Are Ineligible
When assessing opportunity and eligibility in tandem, 4 of 12 potentially eligible patients also had no opportunity for participation. Given the low overall number of eligible patients, maximizing opportunities for trial participation among this group is imperative. The eligibility screening was based solely on protocol eligibility criteria and medical record review. These patients may have been inappropriate for available trials based on information not included in eligibility criteria or the medical record. For example, because the study screening was completed retrospectively, the authors did not have access to information regarding slot availability. Slot availability is particularly important for phase I trials used to determine the recommended dose or schedule of a new drug. Patients often are enrolled in small cohorts, with new slots opening at varying time points dependent on side effects reported by the prior cohort. Patients may have been eligible for participation but were not provided opportunity because no slots were available or waiting for a slot was contraindicated. In addition, these patients may simply have been overlooked.
The fact that most patients fell into the N/I group may indicate that appropriate trials were not available for the new patient population and/or eligibility criteria for existing trials were too stringent. Other studies suggest that lack of trial availability and stringent eligibility critera are barriers to opportunity for CCT enrollment, particularly for under-represented populations (Adams-Campbell et al., 2004; Go et al., 2006; Guadagnolo et al., 2009; Penberthy et al., 2012; Townsley, Selby, & Siu, 2005). Although the authors of the current study were not able to assess this with retrospective eligibility screening, patients in the N/I group may have been pre-screened by healthcare providers or researchers and were either (a) known to be ineligible for all trials or (b) known to not have a slot available to them for a potential trial at the time of their visit. In these situations, a patient may have been considered for trials, but as they could not enroll in any available trials, no opportunity was given.
Increased Opportunity and Eligibility for Patients With Late-Stage Disease
Differences in opportunity and eligibility were strongly associated with cancer stage. This may reflect that the majority of accruing trials were designed for patients with late-stage disease. Available literature supports that patients with advanced disease may be more likely to be enrolled in a trial (Jimenez et al., 2013). However, the sample predominantly included new patients who were early stage and had breast cancer. Simon et al. (2004) also found that patients with early-stage breast cancer were less likely to be offered clinical trials, which they attributed to ineligibility and lack of available CCTs. Patients with early-stage breast cancer also have a variety of efficacious treatments available, which could limit investigators’ interest in opening trials for this group.
Contrary to the literature (Adams-Campbell et al., 2004; Penberthy et al., 2012; Wendler et al., 2006), the current authors found no significant differences in opportunity or eligibility based on race or ethnicity. The current matched groups did not differ based on cancer stage, which proved to be an important predictor of difference in opportunity and eligibility. This factor may be one reason differences were not detected by race or ethnicity. Under-represented populations, including racial and ethnic minority populations, share a disproportionate burden of late-stage disease (Lantz et al., 2006; Virnig et al., 2009). This sample and/or the patients treated at the institution may not be representative of the general under-represented population of patients with cancer. Given that available trials were predominantly designed for patients with late-stage disease, racial and ethnic minority populations may have experienced different, and perhaps better, rates of opportunity and eligibility for CCT participation if the patient population was more representative.
Increased Opportunity for Patients With Medicare or Medicaid
In the current study, patients with Medicare or Medicaid were more likely to have opportunity for CCT participation but were not more likely to be eligible for participation. In 2000, the Clinton administration mandated that the Centers for Medicare and Medicaid Services cover the cost of routine care for Medicare patients on clinical trials. However, this did not include a provision of coverage for Medicaid patients. Patients with Medicare or Medicaid coverage may have presented with later-stage disease and, therefore, had more trials available to them, but because of stringent protocol criteria and/or increased disease burden, were ineligible for participation (Go et al., 2006; Mohd Noor et al., 2013). Go et al. (2006) performed a study in a community cancer setting and found that, although older adults had more trials available to them, they were less likely to be enrolled. Increased opportunity, yet decreased eligibility, may have been related to stringency of protocol inclusion criteria (Go et al., 2006) and could partially explain the current findings.
The literature suggests that opportunity and eligibility for CCT participation is influenced by sociodemographic and disease characteristics. The current authors’ research supports that cancer stage is an important factor. Taken together, the results suggest that opportunity and eligibility vary based on interactions between sociodemographic variables, disease characteristics, and the healthcare setting. Future research should focus on identifying these interactions and how they influence eligibility and opportunity for CCT participation. This may elucidate specific subgroups more at risk for under-representation in CCTs. In addition, research should explore opportunity and eligibility for general cancer populations to see if they differ from new patient populations, as well as the role of slot availability in opportunity for CCT participation.
Limitations
The small sample size may have limited the ability to detect small and medium effects. The study included only new patients with cancer in outpatient clinics within a single hospital in a single city, which may limit the generalizability of the results. The trial portfolio in the study setting, specifically the number and type of trials supported by the CRU, may have limited the findings. Only 40% of Black and Hispanic patients and 48% of Non-Hispanic White patients contacted for study participation were eligible and agreed to participate. Of the 88 participants, 73 agreed to EMR review for eligibility assessment. Higher response rates and higher rates of agreement to EMR review may have influenced the findings. Hispanics are likely to have distinct barriers to opportunity and eligibility that may not be captured in this analysis because of the small number of Hispanic participants (n = 4). These barriers should be assessed in future research with a larger Hispanic population. Response bias may have caused patients to over-report or under-report opportunity for participation. Because patient eligibility screening was completed retrospectively, the authors were not able to consider the role of slot availability as a potential barrier to opportunity for participation. Because most of the participants were ineligible for trials, even if a slot was available, these patients would still not have been able to participate. Finally, additional reasons for ineligibility may exist that were not evaluable or captured within the EMR, resulting in fewer eligible patients.
Implications for Nursing
Increasing CCT participation requires that all patients eligible for available trials are offered enrollment. Evidence shows the use of nurse navigation decreases the time from cancer diagnosis to appropriate treatment, particularly for the underserved (Case, 2011). Preliminary research suggests that oncology nurse navigation may be a cost-effective intervention to improve opportunity for clinical trial enrollment for diverse populations (Holmes, Major, Lyonga, Alleyne, & Clayton, 2012). Oncology nurse navigators may be able to minimize the number of eligible patients missed by healthcare providers in the clinical setting and improve opportunities for CCT participation, particularly for under-represented populations, by flagging patients for eligibility screening and facilitating timely referrals to appropriate trials with available slots for enrollment.
The authors noted that the patients in the sample may not have been representative of the general population of under-represented patients with cancer. Patients from under-represented groups may not have access to NCI–designated institutions and/or choose to receive care in other healthcare settings. Advanced practice nurses have an increasing role in caring for underserved patients in rural and community settings and have a unique opportunity to increase CCT participation for under-represented groups. Research suggests that nurse practitioners are willing to recommend CCT participation to their patients, but would prefer more education surrounding trial benefits and burdens, ethical issues, and the translation of clinical trial evidence into practice (Ulrich et al., 2012). Supporting nurse practitioners’ educational preferences surrounding clinical trials may increase nurse practitioner recommendations and referrals for CCT participation.
Knowledge Translation.
Limited trial availability and ineligibility decrease opportunity for cancer clinical trial (CCT) participation for all populations, including under-represented groups.
Nurse navigators can increase opportunities for CCT participation by facilitating timely referrals to appropriate trials with available slots for enrollment.
Providing advanced practice nurses with educational resources related to CCTs may increase referrals for CCT participation.
Acknowledgments
This research was supported by an NINR F31 Individual Predoctoral Fellowship (1F31NR01407601A1), the Ruth L. Kirschstein T32 predoctoral fellowship (T32NR00710017; PI: Sommers), and the American Nurses Foundation/Council for the Advancement of Nursing Science.
Rearden, Hanlon, Ulrich, Brooks-Carthon, and Sommers contributed to the conceptualization and design, analysis, and manuscript preparation. Rearden completed the data collection. Hanlon provided statistical support.
The authors gratefully acknowledge Alexis Zebrowski, MPH, for her assistance with data collection.
References
- Adams-Campbell LL, Ahaghotu C, Gaskins M, Dawkins FW, Smoot D, Polk OD, DeWitty RL. Enrollment of African Americans onto clinical treatment trials: Study design barriers. Journal of Clinical Oncology. 2004;22:730–734. doi: 10.1200/JCO.2004.03.160. doi:10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- Baggstrom MQ, Waqar SN, Sezhiyan AK, Gilstrap E, Gao F, Morgensztern D, Govindan R. Barriers to enrollment in non-small cell lung cancer therapeutic clinical trials. Journal of Thoracic Oncology. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. doi:10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Tannenbaum SL, Gluck S, Hurley J, Antoni M. Participation in cancer clinical trials: Why are patients not participating? Medical Decision Making. 2014;34:116–126. doi: 10.1177/0272989X13497264. doi:10.1177/0272989X13497264. [DOI] [PubMed] [Google Scholar]
- Case MA. Oncology nurse navigator. Clinical Journal of Oncology Nursing. 2011;15:33–40. doi: 10.1188/11.CJON.33-40. doi:10.1188/11.CJON.33-40. [DOI] [PubMed] [Google Scholar]
- Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. Journal of Clinical Oncology. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- Ford JG, Howerton MW, Bolen S, Gary TL, Lai GY, Tilburt J, Bass EB. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evidence Report/Technology Assessment. 2005;122:1–11. doi: 10.1037/e439572005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. doi:10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- Go RS, Frisby KA, Lee JA, Mathiason MA, Meyer CM, Ostern JL, Umberger KE. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106:426–433. doi: 10.1002/cncr.21597. doi:10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Sutton SK, Pratt C, Gilbertson M, Antonia S, Quinn GP. The bottleneck effect in lung cancer clinical trials. Journal of Cancer Education. 2013;28:488–493. doi: 10.1007/s13187-013-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagnolo BA, Petereit DG, Helbig P, Koop D, Kussman P, Fox Dunn E, Patnaik A. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clinical Trials. 2009;6:610–617. doi: 10.1177/1740774509348526. doi:10.1177/1740774509348526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DR, Major J, Lyonga DE, Alleyne RS, Clayton SM. Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. American Journal of Surgery. 2012;203:415–422. doi: 10.1016/j.amjsurg.2011.02.005. doi:10.1016/j.amjsurg.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Javid SH, Unger JM, Gralow JR, Moinpour CM, Wozniak AJ, Goodwin JW, Albain KS. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316) Oncologist. 2012;17:1180–1190. doi: 10.1634/theoncologist.2011-0384. doi:10.1634/theoncologist.2011-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez R, Zhang B, Joffe S, Nilsson M, Rivera L, Mutchler J, Prigerson HG. Clinical trial participation among ethnic/racial minority and majority patients with advanced cancer: What factors most influence enrollment? Journal of Palliative Medicine. 2013;16:256–262. doi: 10.1089/jpm.2012.0413. doi:10.1089/jpm.2012.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamerus JF, Bruinooge SS, Ye X, Klamerus ML, Damron D, Lansey D, Rudin CM. The impact of insurance on access to cancer clinical trials at a comprehensive cancer center. Clinical Cancer Research. 2010;16:5997–6003. doi: 10.1158/1078-0432.CCR-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford AT, Resnicow K, Dimond EP, Denicoff AM, Germain DS, McCaskill-Stevens W, Go RS. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer. 2014;120:877–884. doi: 10.1002/cncr.28483. doi:10.1002/cncr.28483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, Mujahid M, Schwartz K, Janz NK, Fagerlin A, Salem B, Katz SJ. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. American Journal of Public Health. 2006;96:2173–2178. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman M, Petersen J, Montgomery R. An examination of the influence of patient race and ethnicity on expressed interest in learning about cancer clinical trials. Journal of Cancer Research and Clinical Oncology. 2008;134:115–118. doi: 10.1007/s00432-007-0263-4. [DOI] [PubMed] [Google Scholar]
- Mohd Noor A, Sarker D, Vizor S, McLennan B, Hunter S, Suder A, Papa S. Effect of patient socioeconomic status on access to early-phase cancer trials. Journal of Clinical Oncology. 2013;31:224–230. doi: 10.1200/JCO.2012.45.0999. doi:10.1200/JCO.2012.45.0999. [DOI] [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. doi:10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Cancer health disparities. 2008 Retrieved from http://www.cancer.gov/cancertopics/factsheet/disparities/cancer-health-disparities.
- Penberthy L, Brown R, Wilson-Genderson M, Dahman B, Ginder G, Siminoff LA. Barriers to therapeutic clinical trials enrollment: Differences between African-American and White cancer patients identified at the time of eligibility assessment. Clinical Trials. 2012;9:788–797. doi: 10.1177/1740774512458992. doi:10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, Christian MC. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of Clinical Oncology. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- Simon MS, Du W, Flaherty L, Philip PA, Lorusso P, Miree C, Brown DR. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. Journal of Clinical Oncology. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. doi:10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. Journal of Clinical Oncology. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. doi:10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Zhou Q, Ratcliffe SJ, Ye L, Grady C, Watkins-Bruner D. Nurse practitioners’ attitudes about cancer clinical trials and willingness to recommend research participation. Contemporary Clinical Trials. 2012;33:76–84. doi: 10.1016/j.cct.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: Early-versus late-stage cancer diagnosis. Health Affairs (Millwood) 2009;28:160–168. doi: 10.1377/hlthaff.28.1.160. doi:10.1377/hlthaff.28.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington SF, Luta G, Noone AM, Caicedo L, Lopez-Class M, Sheppard V, Mandelblatt J. Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant Latinos. Journal of Community Health. 2012;37:335–343. doi: 10.1007/s10900-011-9450-y. doi:10.1007/s10900-011-9450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. doi:10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]