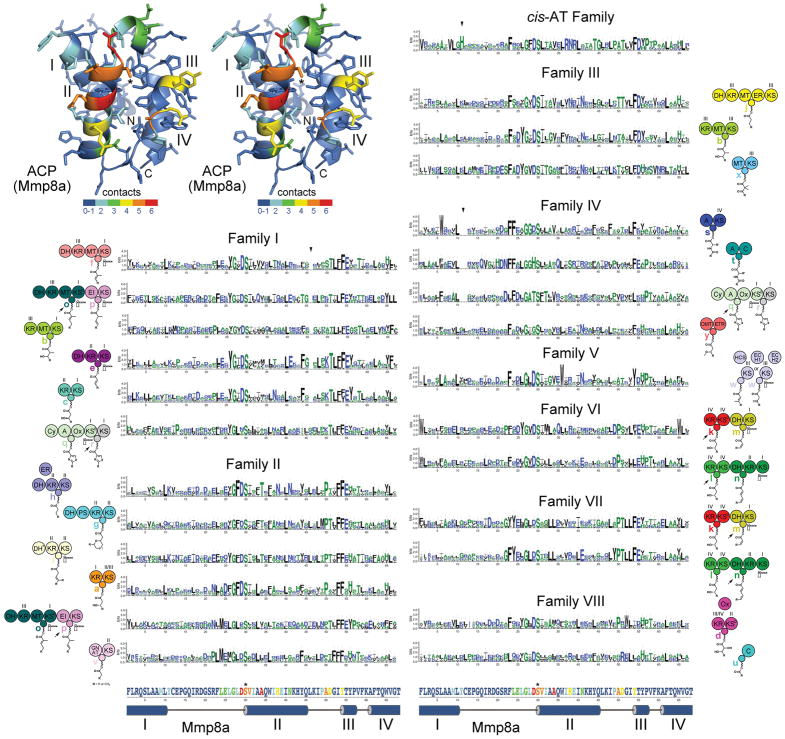
Figure 4. ACP Signatures.
A stereodiagram of an ACP (Mmp8a from the mupirocin trans-AT assembly line, PDB 2L22) shows which positions in assembly line ACPs most frequently contact cognate enzymes in the six available complex structures (red indicates that in 6/6 structures a residue in that position is within 4 Å of the cognate enzyme). The four helices (I–IV), the phosphopantetheinylated serine (*), and the boundaries of the ACP domain (N and C) are marked. Sequence logos show the ACP signatures for each of the module types, which are indicated by the neighboring cartoon. Triangles indicate relative gaps or insertions: in Family I the loop between helices II and III is generally one residue shorter, in Family IV the loop between helices I and II is generally two residues shorter, in cis-AT ACPs the loop between helices I and II is generally one residue longer and contains a conserved histidine. The primary and secondary structure of Mmp8a at the bottom helps show where residues in the ACP signatures are structurally positioned and how likely they are to make contact with cognate enzymes.