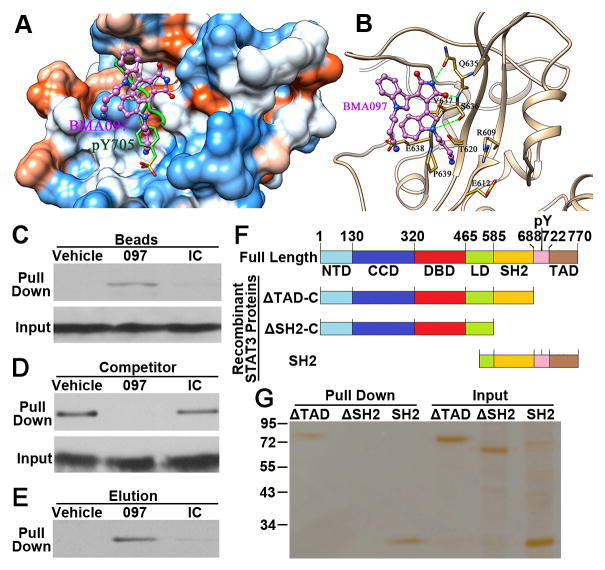
Figure 4. Binding of BMA097 to the SH2 domain of STAT3.
(A–B) The predicted BMA097-binding pocket (A) and binding mode (B) in the SH2 domain of STAT3. Molecular surface in (A) is colored by dodger blue for the most hydrophilic to orange red for the most hydrophobic. The green lines in (B) indicate H-bonds. (C) Pull-down assay. Total lysate from MDA-MB-231 cells were subjected to pull-down assay by CNBr-immobilized BMA097, an irrelevant compound (IC) or vehicle control followed by Western blot analysis of pull-down materials using STAT3 antibody. (D–E) Competition of STAT3-binding to immobilized BMA097 (D) or elution of STAT3 bound to the immobilized BMA097 (E) by excess free BMA097, DMSO vehicle control, or an irrelevant negative control compound (IC). (F) Schematic domain structures of STAT3 and of recombinant proteins. NTD, amino terminal domain; CCD, coiled coil domain; DBD, DNA-binding domain; LD, linker domain; SH2, Src homology 2 domain; TAD, transactivation domain. (G) Pull-down assay of purified recombinant STAT3 proteins with different deletions by immobilized BMA097.