Abstract
Triple inhaled corticosteroid (ICS)/long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) therapy is recommended for symptomatic patients with chronic obstructive pulmonary disease (COPD) and at risk of exacerbations. However, the benefits versus side-effects of triple inhaled therapy for COPD, based on distinct patient clinical profiles, are unclear.
FULFIL, a phase III, randomised, double-blind study, compared 24 weeks of once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 µg using the Ellipta inhaler with twice-daily budesonide/formoterol (BUD/FOR) 400/12 µg using the Turbuhaler. Subgroup analyses of forced expiratory volume in 1 s (FEV1), St George's Respiratory Questionnaire (SGRQ) Total score and exacerbation rates were carried out. Subgroups were defined by COPD medication at screening (ICS+LABA, BUD+FOR, ICS+LABA+LAMA, LAMA alone, tiotropium alone and LAMA+LABA), by disease severity (lung function and exacerbations) and by exacerbation history (exacerbation severity and frequency).
In the intent-to-treat population (n=1810) at week 24, FF/UMEC/VI (n=911) versus BUD/FOR (n=899) improved FEV1 and SGRQ Total score and reduced mean annual exacerbation rates in all disease severity and exacerbation history subgroups. FF/UMEC/VI versus BUD/FOR improved FEV1 and SGRQ Total score in all medication subgroups and reduced mean annual exacerbation rates in all medication subgroups, except LAMA+LABA. Adverse events were similar across subgroups.
These findings support the benefit of FF/UMEC/VI compared with dual ICS/LABA therapy in patients with symptomatic COPD regardless of disease severity or prior treatment and may help to inform clinical decision making.
Short abstract
Single-inhaler triple therapy for COPD provides clinical benefit across a wide spectrum of disease characteristics http://ow.ly/ETBv30iXQ97
Introduction
Triple inhaled pharmacological therapy consisting of an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA) and a long-acting β2-agonist (LABA) is recommended for patients with chronic obstructive pulmonary disease (COPD) with persistent symptoms and at risk of exacerbations [1]. Triple therapy is often used in daily clinical practice, frequently to treat symptoms that continue despite treatment with a LAMA or ICS/LABA [2, 3]. An analysis of patients with COPD in the UK showed that 32% of patients were prescribed triple therapy [4], which was most commonly achieved by adding a LAMA to existing dual therapy (LABA/ICS) [2, 3].
Despite the frequent use of triple therapy, few randomised controlled trials compare the benefits of triple therapy with ICS/LABA for patients with COPD [5]. Triple therapy using ICS/LABA plus a LAMA in patients with COPD improved lung function and disease-specific quality of life (QoL) and reduced hospitalisation rates compared with LAMA plus placebo, with no differences between LAMA/LABA and placebo [6]. The TRINITY study showed triple ICS/LABA/LAMA (beclometasone dipropionate, formoterol fumarate and glycopyrronium bromide (BDP/FoF/GB)) therapy, administered using a single inhaler, reduced exacerbation rates compared with LAMA (tiotropium (TIO)) therapy alone [7]. Comparison of single-inhaler ICS/LABA/LAMA (BDP/FoF/GB) with ICS/LABA (BDP/FoF) in the TRILOGY study showed an improvement in lung function and reduction in exacerbation frequency with triple versus dual therapy, supporting the clinical benefit of adding a LAMA to ICS/LABA [8].
A once-daily single-inhaler triple therapy of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 μg has been developed for patients with moderate to very severe COPD. As well as potentially offering clinical benefit compared with dual ICS/LABA therapy, triple therapy delivered using a single inhaler eliminates the need for multiple inhalers and may also help to ensure that the prescribed combination is delivered consistently.
The FULFIL (Lung FUnction and quality of LiFe assessment in COPD with closed trIpLe therapy) study was the first to compare once-daily single-inhaler triple therapy (FF/UMEC/VI) with twice-daily budesonide/formoterol (BUD/FOR) in patients with symptomatic COPD [9]. FULFIL showed statistically significant and clinically meaningful improvements in lung function (forced expiratory volume in 1 s (FEV1)) and health-related QoL (HRQoL; St George's Respiratory Questionnaire (SGRQ)) and reduced exacerbation rates with FF/UMEC/VI versus BUD/FOR. The results from FULFIL also showed that the safety profile of FF/UMEC/VI was in line with that of the components and no new safety signals emerged [9].
The FULFIL study included patients with COPD with differing levels of disease severity, treatment backgrounds and exacerbation histories in an effort to closely reflect daily clinical practice. Understanding the response to triple therapy in specific patient subgroups within the broader overall population may help to inform clinicians when choosing inhaled therapies. These subgroup analyses evaluated the effects of prior COPD medication, disease severity and exacerbation history on the relative efficacy of FF/UMEC/VI compared with BUD/FOR to improve lung function, HRQoL and the annual rate of moderate and severe exacerbations.
Methods
FULFIL was a phase III, randomised, double-blind, double-dummy, parallel-group, multicentre study (ClinicalTrials.gov identifier NCT02345161; GSK study number CTT116853) and the design has been described previously [9]. Patients in the intent-to-treat (ITT) population were randomised to receive once-daily FF/UMEC/VI 100/62.5/25 µg using the Ellipta inhaler or twice-daily BUD/FOR 400/12 µg using the Turbuhaler for 24 weeks. A subset of patients from the ITT population received blinded study treatment for up to 52 weeks (extension (EXT) population). The coprimary end-points were change from baseline in trough FEV1 and change from baseline in SGRQ Total score at week 24. Exacerbation rate was a secondary end-point. The results from these analyses in the overall ITT population are reported elsewhere [9]. Subgroup analyses of FEV1, SGRQ Total score and exacerbation rates during FULFIL by prior COPD medication class, disease severity and exacerbation history were carried out. These analyses were pre-specified, aside from the LAMA+LABA prior COPD medication and the exacerbation subgroups, which were post hoc.
The institutional review boards for human studies provided their required approvals of the protocol and written informed consent was obtained from the patients or their surrogates.
Patients
Patients enrolled in FULFIL were aged ≥40 years with advanced symptomatic COPD (FEV1 % pred <50% and COPD Assessment Test (CAT) score ≥10, or FEV1 % pred 50– <80%, CAT ≥10 with either ≥2 moderate exacerbations per year or ≥1 severe exacerbations per year). Patients were required to have been on maintenance COPD medication for ≥3 months prior to screening. During the 2-week run-in period, patients were required to remain on the same medication, only stopping when they started randomised treatment. Medical history, current medications, lung function screening, and COPD and exacerbation history were collected during the screening visit, based on patient reports.
Patient subgroups based on common disease characteristics and treatments (supplementary table S1) were defined by COPD medication class at screening, disease severity at screening (based on lung function and history of exacerbations in the 12 months prior to screening) and exacerbation history in the 12 months prior to screening.
The COPD medication class subgroups were: ICS+LABA, BUD+FOR, ICS+LABA+LAMA, LAMA alone, TIO alone and LAMA+LABA. The disease severity subgroups were: FEV1 % pred <50%, no moderate/severe exacerbations; FEV1 % pred <50%, ≥1 moderate/severe exacerbations; and FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations. The exacerbation history subgroups were: 0/1 moderate exacerbations, ≥2 moderate exacerbations (no severe exacerbations) and ≥1 severe exacerbations. Further subgroups (FEV1 % pred <50% and ≥2 moderate/≥1 severe exacerbations, FEV1 % pred <50% and 1 moderate exacerbation, FEV1 % pred ≥50%, FEV1 % pred 30– <50%, and FEV1 % pred <30%) were also assessed and the results are presented in the supplementary material.
Efficacy assessments
The efficacy assessments used in this study have been described previously [9]; in brief, spirometry was performed in the ITT population at baseline and at weeks 2, 4, 12 and 24, and at weeks 36 and 52 in the EXT population, according to the American Thoracic Society/European Respiratory Society criteria [10]. The SGRQ for COPD patients was completed by patients using an e-diary at day 1 and at weeks 4 and 24 (and week 52 for the EXT population). Potential COPD exacerbations were identified based on symptoms reported using the e-diary and were defined as: mild, worsening symptoms of COPD that were self-managed by the patient (e.g. increase in albuterol use) and not associated with the use of corticosteroids or antibiotics; moderate, worsening symptoms of COPD that required treatment with systemic corticosteroids and/or antibiotics; and severe, worsening symptoms of COPD that required treatment with in-patient hospitalisation.
Safety assessments
The safety assessments in FULFIL have been described previously [9]; in brief, incidence of adverse events, serious adverse events and adverse events of special interest was evaluated during the study. Further safety analyses were carried out for two patient subgroups defined by exacerbation history in the previous 12 months: <2 moderate exacerbations and ≥2 moderate or ≥1 severe exacerbations.
Statistical analyses
The ITT population included all randomised patients, except those who were randomised in error. The EXT population included a subset of patients in the ITT population who received treatment for up to 52 weeks. Patients in the EXT population were included in analyses of the ITT population up to week 24. Least squares mean changes from baseline and mean treatment differences between FF/UMEC/VI and BUD/FOR in trough FEV1 and SGRQ Total score at each scheduled visit in each subgroup category were calculated from a mixed model repeated measures analysis (covariates: treatment group, smoking status, geographical region, visit, baseline value, baseline by visit and treatment by visit interactions). The number of moderate or severe exacerbations up to week 24 in each subgroup category was analysed using a generalised linear model assuming a negative binomial distribution. Covariates included treatment group, exacerbation history (0, 1, ≥2 moderate or severe exacerbations in the 12 months prior to screening), smoking status, geographical region and FEV1 % pred at baseline, with logarithm of time on treatment as an offset variable. Mean annual exacerbation rates, FF/UMEC/VI versus BUD/FOR rate ratios and percentage rate reductions were calculated.
Results
The ITT population for FULFIL included 1810 patients [9], who were randomised to receive FF/UMEC/VI (n=911) or BUD/FOR (n=899). The first 430 patients enrolled in FULFIL were included in the EXT population (FF/UMEC/VI, n=210; BUD/FOR, n=220) that received study treatment for up to 52 weeks. The distribution of patients within each subgroup was well balanced between arms and for each category (table 1).
TABLE 1.
Distribution of patients within subgroups in the intent-to-treat (ITT) population (which included the extension (EXT) population up to week 24) and the EXT population (up to week 52)
| Week 24 (ITT population) | Week 52 (EXT population) | |||||
| FF/UMEC/VI | BUD/FOR | Total | FF/UMEC/VI | BUD/FOR | Total | |
| Total patients | 911 | 899 | 1810 | 210 | 220 | 430 |
| COPD medication class at screening# | ||||||
| Patients | 705 | 678 | 1383 | 181 | 171 | 352 |
| ICS+LABA | 268 (29) | 259 (29) | 527 (29) | 72 (34) | 68 (31) | 140 (33) |
| BUD+FOR | 87 (10) | 83 (9) | 170 (9) | 28 (13) | 25 (11) | 53 (12) |
| ICS+LABA+LAMA | 257 (28) | 256 (28) | 513 (28) | 63 (30) | 58 (26) | 121 (28) |
| LAMA alone | 79 (9) | 79 (9) | 158 (9) | 20 (10) | 20 (9) | 40 (9) |
| TIO alone | 65 (7) | 67 (7) | 132 (7) | 15 (7) | 16 (7) | 31 (7) |
| LAMA+LABA | 101 (11) | 84 (9) | 185 (10) | 26 (12) | 25 (11) | 51 (12) |
| Disease severity | ||||||
| Patients | 906 | 894 | 1800 | 208 | 218 | 426 |
| FEV1 % pred <50%, no moderate/severe exacerbations | 311 (34) | 315 (35) | 626 (35) | 62 (30) | 71 (32) | 133 (31) |
| FEV1 % pred <50%, ≥1 moderate/severe exacerbation | 299 (33) | 290 (32) | 589 (33) | 70 (33) | 73 (33) | 143 (33) |
| FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations | 296 (33) | 289 (32) | 585 (33) | 76 (36) | 74 (34) | 150 (35) |
| Exacerbation history | ||||||
| Patients | 911 | 899 | 1810 | 210 | 220 | 430 |
| 0/1 moderate exacerbations | 600 (66) | 613 (68) | 1213 (67) | 148 (70) | 159 (72) | 307 (71) |
| ≥2 moderate exacerbations | 311 (34) | 286 (32) | 597 (33) | 62 (30) | 61 (28) | 123 (29) |
| ≥1 severe exacerbations | 185 (20) | 200 (22) | 385 (21) | 58 (28) | 57 (26) | 115 (27) |
Data are presented as n or n (%). FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg); COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; TIO: tiotropium; FEV1: forced expiratory volume in 1 s. #: subgroups are not exclusive (the n-values in the “Patients” row represent the number of patients in any of the categories for the subgroup; patients receiving BUD/FOR are included in the ICS+LABA group and patients receiving TIO alone are included in the LAMA alone group).
Effect of prior COPD medication class
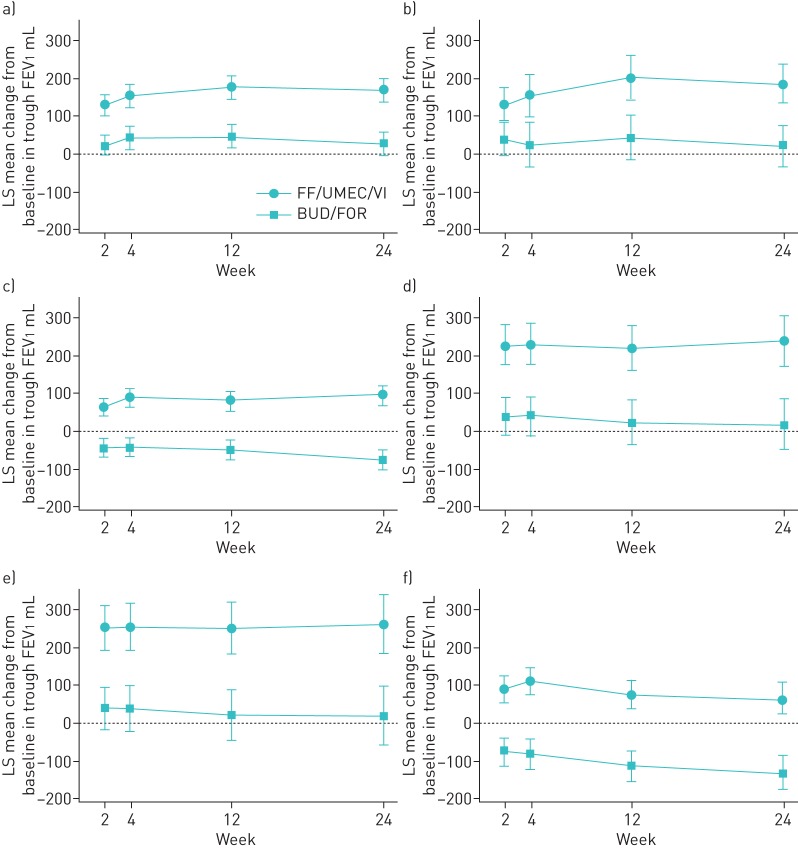
Treatment with FF/UMEC/VI compared with BUD/FOR improved trough FEV1 irrespective of the class of prior COPD medication received. At week 24, least squares mean change from baseline in trough FEV1 improved by between 145 and 242 mL with FF/UMEC/VI compared with BUD/FOR and the difference from BUD/FOR was statistically significant (p<0.01) for each class of medication at each visit (figure 1). A similar response was observed at week 52 in the EXT population, with statistically significant improvements in least squares mean change from baseline in trough FEV1 in all subgroups with FF/UMEC/VI (range 162–270 mL) compared with BUD/FOR (supplementary table S2).
FIGURE 1.
Least squares (LS) mean change from baseline (95% CI) in trough forced expiratory volume in 1 s (FEV1) according to previous chronic obstructive pulmonary disease medication (intent-to-treat population). ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; BUD: budesonide; FOR: formoterol; LAMA: long-acting muscarinic antagonist; TIO: tiotropium; FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) ICS+LABA, b) BUD+FOR, c) ICS+LABA+LAMA, d) LAMA alone, e) TIO alone and f) LAMA+LABA.
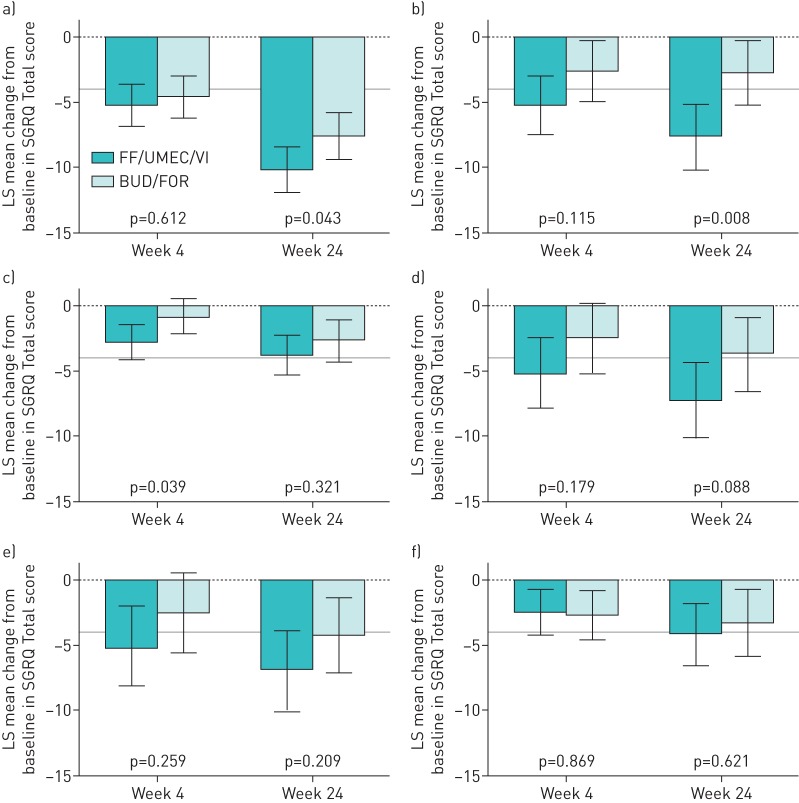
FF/UMEC/VI also improved SGRQ Total score compared with BUD/FOR in all prior medication subgroups. The least squares mean change from baseline in SGRQ Total score improved by −1.1 to −4.8 with FF/UMEC/VI and the difference from BUD/FOR was statistically significant (p=0.043) for the ICS+LABA prior treatment subgroup at week 24 (figure 2). Although improvements were seen in SGRQ Total score with FF/UMEC/VI compared with BUD/FOR in all subgroups, except ICS+LABA and TIO alone, none were statistically significant at week 52 in the EXT population (supplementary table S2).
FIGURE 2.
Least squares (LS) mean change from baseline (95% CI) in St George's Respiratory Questionnaire (SGRQ) Total score according to previous chronic obstructive pulmonary disease medication subgroups (intent-to-treat population). ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; BUD: budesonide; FOR: formoterol; LAMA: long-acting muscarinic antagonist; TIO: tiotropium; FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) ICS+LABA, b) BUD+FOR, c) ICS+LABA+LAMA, d) LAMA alone, e) TIO alone and f) LAMA+LABA. The grey line indicates the 4-unit change in SGRQ score considered the minimal clinically important difference.
FF/UMEC/VI compared with BUD/FOR reduced the mean annual exacerbation rate up to week 24 (range 24–63%) in all prior medication subgroups, except LAMA+LABA (annual exacerbation rate reduction −44%) (table 2). The ratio of FF/UMEC/VI:BUD/FOR was statistically significant in the ICS+LABA (0.37, 95% CI 0.20–0.71; p=0.003) and ICS+LAMA+LABA (0.53, 95% CI 0.33–0.87; p=0.012) prior treatment subgroups.
TABLE 2.
Mean annual moderate/severe exacerbation rates by subgroup (intent-to-treat (ITT) population; up to week 24)
| Subgroup | FF/UMEC/VI | BUD/FOR | Rate reduction % (95% CI) | ||
| n | Rate | n | Rate | ||
| Patients | 911 | 899 | |||
| Prior medication | |||||
| ICS+LABA | 266 | 0.10 | 258 | 0.27 | 63 (29–80)*** |
| BUD+FOR | 87 | 0.05 | 83 | 0.10 | 54 (−74–88) |
| ICS+LABA+LAMA | 256 | 0.28 | 254 | 0.53 | 47 (13–67)* |
| LAMA alone | 79 | 0.12 | 79 | 0.23 | 49 (−42–81) |
| TIO alone | 65 | 0.15 | 67 | 0.20 | 24 (−125–74) |
| LAMA+LABA | 100 | 0.38 | 83 | 0.26 | −44 (−194–29) |
| Disease severity | |||||
| FEV1 % pred <50%, no moderate/severe exacerbations | 311 | 0.22 | 315 | 0.33 | 33 (−4–57) |
| FEV1 % pred <50%, ≥1 moderate/severe exacerbation | 299 | 0.22 | 290 | 0.41 | 45 (11–66)* |
| FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations | 296 | 0.22 | 289 | 0.30 | 27 (−21–56) |
| Exacerbation history | |||||
| 0/1 moderate exacerbations | 599 | 0.25 | 609 | 0.40 | 36 (11–54)** |
| ≥2 moderate exacerbations | 308 | 0.24 | 283 | 0.32 | 25 (−23–55) |
| ≥1 severe exacerbations | 185 | 0.13 | 198 | 0.32 | 59 (17–80)* |
FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg); ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; TIO: tiotropium; FEV1: forced expiratory volume in 1 s. *: p<0.05; **: p<0.01; ***: p<0.005, statistically significant difference for FF/UMEC/VI:BUD/FOR ratio.
Results for additional prior medication subgroups (LABA only and not receiving ICS+LABA+LAMA) are presented in supplementary table S3.
Effect of disease severity
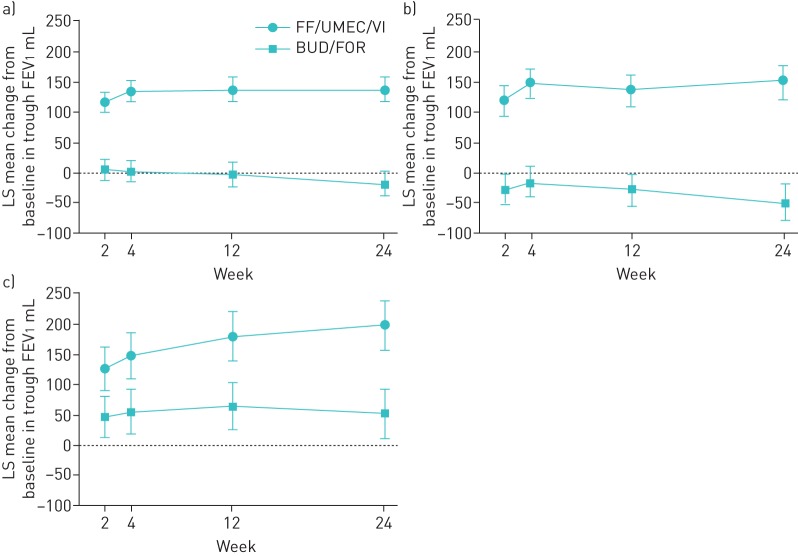
Treatment with FF/UMEC/VI compared with BUD/FOR significantly improved trough FEV1 irrespective of disease severity. At week 24, the improvement in trough FEV1 for FF/UMEC/VI compared with BUD/FOR in the three disease severity subgroups was 178 mL (95% CI 0.14–0.22) for FEV1 <50% predicted, no moderate/severe exacerbations; 118 mL (95% CI 0.08–0.16) for FEV1 % pred <50%, ≥1 moderate/severe exacerbations; and 215 mL (95% CI 176–255) for FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations. These treatment differences were all statistically significant (all groups p<0.001) (figure 3). The findings were similar at week 52; the improvement in trough FEV1 (range 140–224 mL) with FF/UMEC/VI compared with BUD/FOR was statistically significant (p<0.001) in all three subgroups (supplementary table S2).
FIGURE 3.
Least squares (LS) mean change from baseline (95% CI) in trough forced expiratory volume in 1 s (FEV1) according to chronic obstructive pulmonary disease severity (intent-to-treat population). FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) FEV1 % pred <50%, no moderate/severe exacerbations (n=626), b) FEV1 % pred <50%, ≥1 moderate/severe exacerbations (n=589) and c) FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations (n=585).
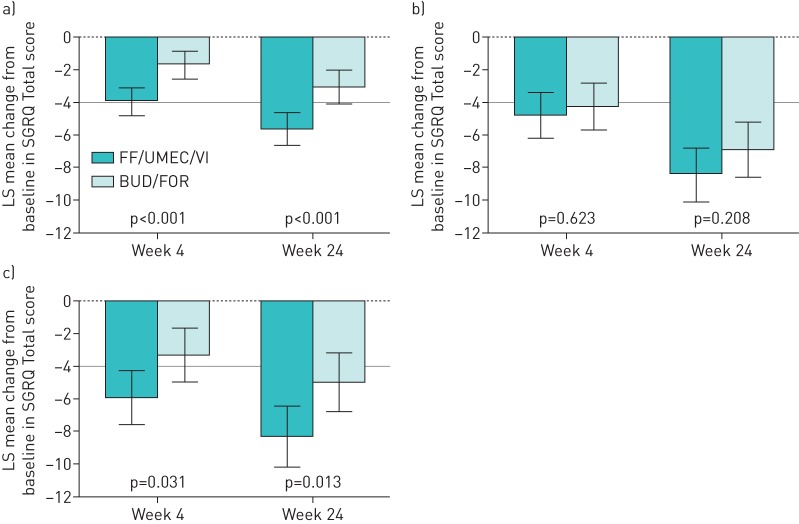
Improvements in SGRQ Total scores were seen with FF/UMEC/VI compared with BUD/FOR, irrespective of disease severity. The improvement in SGRQ Total scores at week 24 for FF/UMEC/VI compared with BUD/FOR in the three subgroups was −1.9 (95% CI −4.1–0.3) for FEV1 <50% predicted, no moderate/severe exacerbations; −2.5 (95% CI −4.7– −0.3) for FEV1 % pred <50%, ≥1 moderate/severe exacerbations; and −2.2 (95% CI −4.4–0.0) for FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations. The improvement was statistically significant (p<0.05) for the groups with FEV1 % pred <50%, ≥1 moderate/severe exacerbations and FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations (figure 4). At week 52 in the EXT population, improvements were seen with FF/UMEC/VI compared with BUD/FOR in the three subgroups, but were not statistically significant (supplementary table S2).
FIGURE 4.
Least squares (LS) mean change from baseline (95% CI) in St George's Respiratory Questionnaire (SGRQ) Total score according to chronic obstructive pulmonary disease severity (intent-to-treat population). FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) Forced expiratory volume in 1 s (FEV1) % pred <50%, no moderate/severe exacerbations (n=626), b) FEV1 % pred <50%, ≥1 moderate/severe exacerbations (n=589) and c) FEV1 % pred 50– <80%, ≥2 moderate or ≥1 severe exacerbations (n=585). The grey line indicates the 4-unit change in SGRQ score considered the minimal clinically important difference.
There was a reduction in the mean annual exacerbation rates in all disease severity subgroups up to week 24 (range 45–27%) for FF/UMEC/VI compared with BUD/FOR (table 2). The FF/UMEC/VI:BUD/FOR ratio of the annual rate (0.55, 95% CI 0.34–0.89) was statistically significant (p=0.015) in the FEV1 % pred <50%, ≥1 moderate/severe exacerbations subgroup (group 2).
Results for additional disease severity subgroups are presented in supplementary table S3.
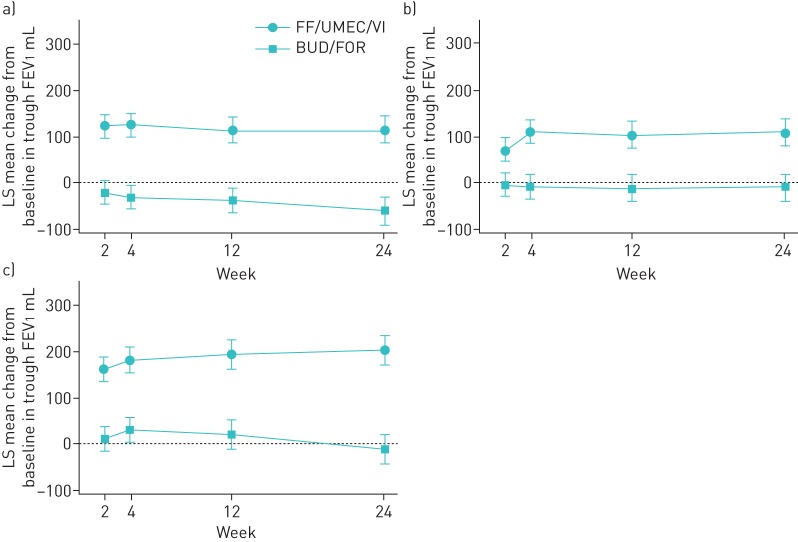
Effect of exacerbation history
Treatment with FF/UMEC/VI improved trough FEV1 compared with BUD/FOR regardless of exacerbation history (figure 5). At week 24, the improvement in trough FEV1 for FF/UMEC/VI compared with BUD/FOR was 157 mL (95% CI 129–186) in the 0/1 moderate exacerbations group, 199 mL (95% CI 158–240) in the ≥2 moderate exacerbations group and 146 mL (95% CI 87–205) in the ≥1 severe exacerbations group; all differences were statistically significant (p<0.001). At week 52, FF/UMEC/VI also statistically significantly (at least p<0.05) improved trough FEV1 compared with BUD/FOR in all three subgroups (supplementary table S2).
FIGURE 5.
Least squares (LS) mean change from baseline (95% CI) in trough forced expiratory volume in 1 s (FEV1) according to exacerbation history (intent-to-treat population). FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) 0/1 moderate exacerbations, b) ≥2 moderate exacerbations and c) ≥1 severe exacerbations.
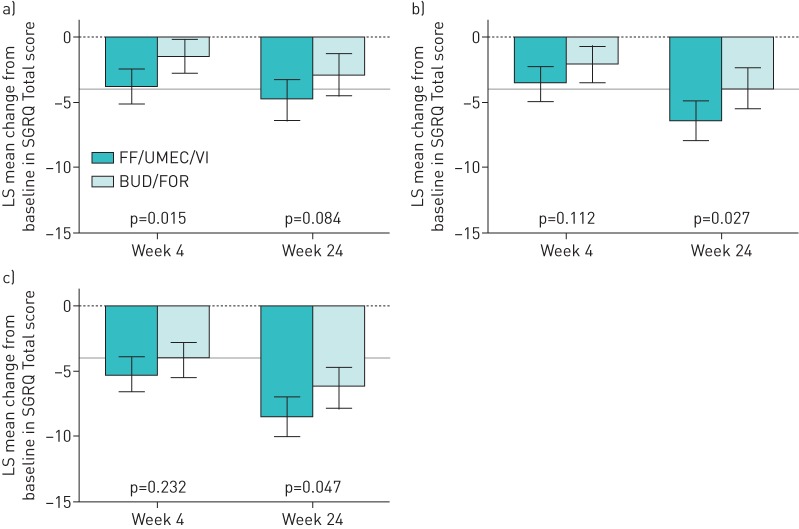
SGRQ Total score improved with FF/UMEC/VI compared with BUD/FOR at week 24 in all three exacerbation history subgroups; however, the difference was statistically significant in the 0/1 moderate exacerbations group (−2.6, 95% CI −4.0– −1.1; p<0.001) and the ≥1 severe exacerbations group (−3.3, 95% CI −5.9– −0.7; p=0.013), but not the ≥2 moderate exacerbations group (−1.5, 95% CI −3.9–0.9; p=0.208) (figure 6). Improvements in SGRQ Total score with FF/UMEC/VI compared with BUD/FOR were observed in the three groups at week 52; however, only the difference between treatments in the ≥2 moderate exacerbations group was statistically significant (p=0.026) (supplementary table S2).
FIGURE 6.
Least squares (LS) mean change from baseline (95% CI) in St George's Respiratory Questionnaire (SGRQ) Total score according to exacerbation history (intent-to-treat population). FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol (100/62.5/25 µg); BUD/FOR: budesonide/formoterol (400/12 µg). a) 0/1 moderate exacerbations, b) ≥2 moderate exacerbations and c) ≥1 severe exacerbations. The grey line indicates the 4-unit change in SGRQ score considered the minimal clinically important difference.
There was a reduction in mean annual exacerbation rates in all exacerbation history subgroups with FF/UMEC/VI compared with BUD/FOR (range 57–27%) up to week 24 (table 2). The ratio of FF/UMEC/VI:BUD/FOR was statistically significant in the 0/1 moderate exacerbations (0.64, 95% CI 0.46–0.89; p=0.008) and the ≥1 severe exacerbations groups (0.41, 95% CI 0.20–0.83; p=0.013).
Safety
The analysis of safety in the ITT population has been published previously [9], and showed there were no emerging safety signals and the profile of FF/UMEC/VI reflected that of the components. The analysis of safety by exacerbation history (<2 moderate exacerbations and ≥2 moderate or ≥1 severe exacerbations in the previous 12 months) showed little variation between the two groups; the most common events were nasopharyngitis and headache in both (supplementary table S4). These results reflect the findings of the ITT population.
Discussion
This subgroup analysis showed that FF/UMEC/VI compared with BUD/FOR improved lung function, as measured by trough FEV1, and HRQoL, as measured by SGRQ Total score, irrespective of disease severity, exacerbation history and prior medication. In addition, reductions in annual moderate/severe exacerbations rates were seen with FF/UMEC/VI compared with BUD/FOR irrespective of disease severity and exacerbation history. FF/UMEC/VI reduced annual moderate/severe exacerbations rates compared with BUD/FOR in all prior medication subgroups, except patients who had been treated with LAMA+LABA. FF/UMEC/VI also improved trough FEV1 and SGRQ Total score compared with baseline in patients who had been receiving ICS+LABA+LAMA as their prior medication.
Although the differences between FF/UMEC/VI and BUD/FOR were statistically significant for FEV1 in all the patient subgroups at both week 24 (ITT population) and week 52 (EXT population), the differences between treatments in SGRQ Total score and exacerbation rate were only statistically significant for some of the prior COPD treatment, disease severity and exacerbation history subgroups, although the improvements generally favoured FF/UMEC/VI. This may be partially due to the low numbers of patients in some of these groups.
The statistically significant improvements in SGRQ Total score with FF/UMEC/VI compared with BUD/FOR were observed in the subgroups with more severe disease (defined by FEV1 and exacerbation frequency), but not in the group with fewest/least severe previous exacerbations. One potential explanation for some of these findings is that the impact of FF/UMEC/VI compared with BUD/FOR on HRQoL may be larger in patients with more severe disease who potentially have greater capacity for improvement. A statistically significant improvement in SGRQ Total score with FF/UMEC/VI compared with BUD/FOR was also observed in the groups that had received BUD+FOR or ICS+LABA previously, supporting the findings from the overall analysis of FULFIL. The improvement in SGRQ Total score with FF/UMEC/VI compared with BUD/FOR was not statistically significant in other prior medication subgroups, possibly because the numbers of patients in these groups were small.
Statistically significant reductions in exacerbation rates with FF/UMEC/VI compared with BUD/FOR were recorded in the subgroups that had previously received ICS+LABA, supporting the benefit of the addition of LAMA, and ICS+LABA+LAMA. However, in the group that previously received LAMA+LABA, the reduction in exacerbation rates favoured treatment with BUD/FOR. Interestingly, statistically significant reductions in exacerbation rates with FF/UMEC/VI compared with BUD/FOR were observed in the FEV1 % pred <50%, ≥1 moderate/severe exacerbations disease severity subgroup and also in the 0/1 moderate and the ≥1 severe exacerbations subgroups. It is not clear why the difference in exacerbation rate in the LAMA+LABA group was numerically in the opposite direction to the other prior medication subgroups. This was not the case in the FLAME trial (ClinicalTrials.gov identifier NCT01782326), which showed that treatment with LAMA/LABA was statistically significantly more effective in preventing COPD exacerbations than treatment with LAMA and an inhaled glucocorticoid [11]. It is also unclear why the reduction in exacerbation rates with FF/UMEC/VI compared with BUD/FOR in the disease severity and exacerbation history subgroups does not appear to follow a consistent pattern. However, the relatively small number of patients in the subgroups could have contributed to this disparity and accentuated between-group differences.
Results from other studies have also shown improvements in lung function and HRQoL as well as reducing exacerbation rates with the addition of LAMA to ICS/LABA in patients with COPD [8, 12]. The consistent improvement in lung function and HRQoL regardless of prior medication suggests that the benefits of FF/UMEC/VI observed here are not a consequence of patients who were randomised to receive BUD/FOR demonstrating a “stepping-down” effect from prior triple therapy.
FULFIL included patients with comorbidities and the study design incorporated a run-in period (when patients remained on their usual medications), meaning these results may be more readily applicable to daily clinical practice than other more restrictive studies. FULFIL compared FF/UMEC/VI with BUD/FOR, which are administered using different dosing regimens and different inhalers; however, the double-dummy design mitigated some of these differences, enabling a more direct comparison of the products.
In conclusion, these subanalyses have shown that reductions in mean annual exacerbation rates with once-daily FF/UMEC/VI compared with twice-daily BUD/FOR were observed in all patients regardless of disease severity or exacerbation history and all prior COPD medication class subgroups, except for LAMA+LABA, and improvements in FEV1 and SGRQ Total score occurred regardless of prior treatment or disease severity.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1–S4 00119-2017_supplementary (220.9KB, pdf)
Acknowledgements
We would like to thank the patients and their families for participating in this study, Helen Barnacle, Rajat Mohindra, Eva Gomez (Operations Lead) and Niki Day (Clinical Safety Scientist) (all at GSK, Uxbridge, UK), Erik Steinberg (Data Quality Leader; GSK, Centennial, CO, USA), and the FULFIL study team. We would also like to thank Veramed (London, UK) for support with statistical analyses.
Prior to the acceptance of the manuscript, medical writing support in the form of development of the draft outline and manuscript drafts in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, referencing and graphic services was provided by Alison Scott (Gardiner-Caldwell Communications, Macclesfield, UK) and was funded by GSK.
This study has previously been presented in abstract form at the 2017 ERS International Congress (Milan, Italy) [13] and the 2017 British Thoracic Society Winter Meeting (London, UK) [14].
Footnotes
This article has supplementary material available from openres.ersjournals.com
This study is registered at ClinicalTrials.gov with identifier number NCT02345161.
Conflict of interest: D.M.G. Halpin reports receiving personal fees from GSK during the conduct of the study, and personal fees and nonfinancial support from AstraZeneca, Boehringer Ingelheim and Novartis; and personal fees from Pfizer and Chiesi, outside the submitted work. R. Birk is an employee of GSK and holds shares in that company. N. Brealey is an employee of GSK and holds shares in that company. G. Criner reports receiving consulting fees from GSK and performing a contracted clinical trial for the company during the conduct of the study. He reports receiving grants from the US NIH and the Dept of Defense; receiving consulting fees from and performing contracted clinical trials for AstraZeneca, Boehringer Ingelheim, Avisa, Mereo, PneumRx/BTG, Pulmonx, Broncus and Lungpacer; receiving consulting fees from Holaira, Third Pole, Pearl, Amirall and CSA Medical; and performing contracted clinical trials for Novartis and Yungjin, all outside the submitted work. M.T. Dransfield reports receiving consulting fees from GSK and performing a contracted clinical trial for the company during the conduct of the study. He reports receiving grants from the US National Institutes of health and the Dept of Defense; receiving consulting fees from and performing contracted clinical trials for AstraZeneca, Boehringer Ingelheim and Boston Scientific; receiving consulting fees from Genentech; and performing contracted clinical trials for Novartis, Pulmonx, PneumRx/BTG and Yungjin, all outside the submitted work. E. Hilton is an employee of GSK and holds shares in that company. D.A. Lomas reports receiving grants, personal fees and nonfinancial support from GSK during the conduct of the study; personal fees and grants from GSK; and personal fees from Griffols, all outside the submitted work. C-Q. Zhu is an employee of GSK and holds shares in that company. D.A. Lipson is an employee of GSK and holds shares in that company.
Support statement: This study was funded by GSK (ClinicalTrials.gov identifier NCT02345161; GSK study number CTT116853). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2017. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ Date last accessed: July 31, 2017.
- 2.Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS One 2014; 9: e105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simeone JC, Luthra R, Kaila S, et al. . Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis 2017; 12: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brusselle G, Price D, Gruffydd-Jones K, et al. . The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis 2015; 10: 2207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siler TM, Kerwin E, Tombs L, et al. . Triple therapy of umeclidinium+inhaled corticosteroids/long-acting beta2 agonists for patients with COPD: pooled results of randomized placebo-controlled trials. Pulm Ther 2016; 2: 43–58. [Google Scholar]
- 6.Aaron SD, Vandemheen KL, Fergusson D, et al. . Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146: 545–555. [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Papi A, Corradi M, et al. . Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 8.Singh D, Papi A, Corradi M, et al. . Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 9.Lipson D, Barnacle H, Birk R, et al. . FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 438–446. [DOI] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 11.Wedzicha JA, Banerji D, Chapman KR, et al. . Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med 2016; 373: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 12.Siler TM, Kerwin E, Sousa AR, et al. . Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med 2015; 109: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 13.Lipson D, Barnacle H, Birk R, et al. . Single inhaler triple therapy in advanced COPD patients: prior medication and disease severity FULFIL subanalyses. Eur Respir J 2017; 50: Suppl. 61, PA1057. [Google Scholar]
- 14.Hilton E, Brealey N, Birk R, et al. . Improvements in exacerbation rates with single inhaler triple therapy versus dual ICS/LABA therapy in patients with advanced chronic obstructive pulmonary disease (COPD): subgroup analyses of the phase III FULFIL study. Thorax 2017; 72: Suppl. 3, P272. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1–S4 00119-2017_supplementary (220.9KB, pdf)