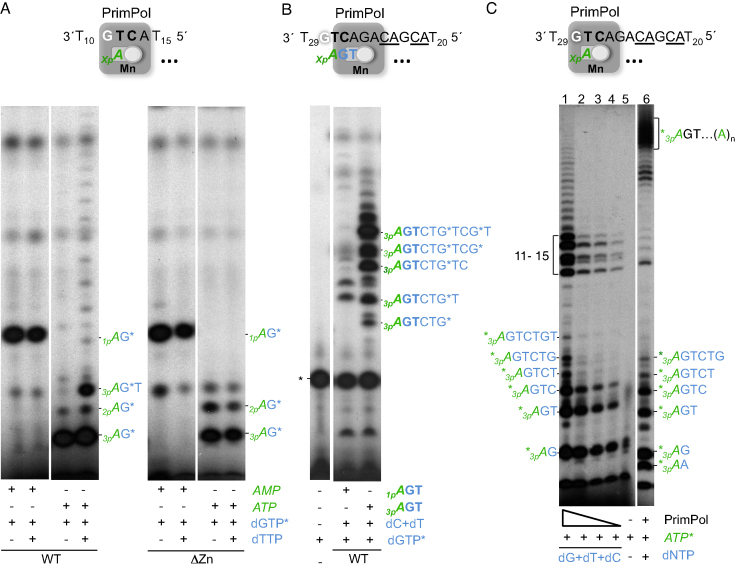
Figure 6.
A putative interaction of PrimPol ZnFD with the 5′-end triphosphate is required for dimer translocation and processive elongation. (A) Representative primase assay (n = 3) using the oligo GTCA (1 μM) to measure dimer formation either by wild-type PrimPol (WT) or its ΔZnFD mutant (ΔZn) at 400 nM, in the presence of [α-32P]dGTP (16 nM) and either AMP (100 μM) or ATP (10 μM for the WT; 100 μM for the ΔZn). When indicated, dTTP (10 μM) was added to evaluate elongation of the dimers. (B) Elongation of a synthetic miniprimer (1pAGT or 3pAGT; 10 μM) in the presence of dCTP, dTTP (10 μM) and [α-32P]dGTP (16 nM) by wild-type PrimPol (WT) at 400 nM. *indicates a main labeled product, intrinsic to [α-32P]dGTP that is present in all conditions including the control without enzyme. (C) Lanes 1–4: evaluation of processive primer synthesis by WT PrimPol (400, 200, 100, 50 nM), in the presence of [γ-32P]ATP (16 nM) and 10 μM (dG/dT/dC)TP. A control reaction in the absence of PrimPol and dNTPs (lane 5) shows minor products intrinsic to the batch of labeled ATP. Addition of the four dNTPs at 10 μM, [γ-32P]ATP (16 nM) and WT PrimPol (400 nM) allowed full extension of the primers (lane 6). The autoradiographs shown are representative of 4 independent experiments.