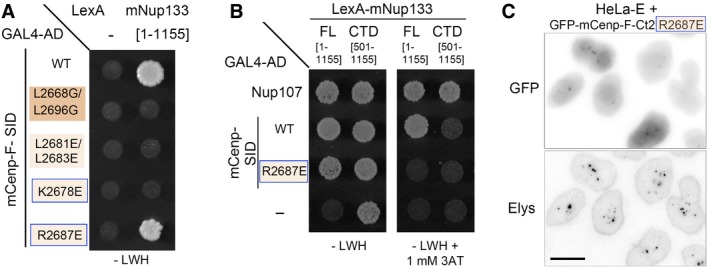
Y2H interactions between LexA alone (−) or fused to mNup133 full‐length (FL, [aa 1–1,155]) or C‐terminal domain (CTD, [aa 501–1,155]) and GAL4‐AD either alone (−) or fused to mCenp‐F‐SID (WT or bearing the indicated mutations) or Nup107 [aa 784–925] were assayed based on growth on ‐LWH medium supplemented, when indicated, by 1 mM 3‐Aminotriazole (3AT). Note in (A) that the
mCenp‐FR2687E mutant, but none of the other mCenp‐F mutants assayed in Fig
2B, interacts with full‐length mNup133. However, unlike WT mCenp‐F‐SID, the interaction of the R2687E mutant with full‐length mNup133 is abrogated by the addition of 1 mM 3‐Aminotriazole, indicating that the
mCenp‐FR2687E mutant may interact with the full‐length mNup133 in a rather weak manner. In (B), note that the lexA‐mNup133‐CTD construct is transactivating when used in ‐LWH medium. Under conditions required to prevent this transactivation (i.e., addition of 1 mM 3‐Aminotriazole), the interaction of Nup133‐Cterm with its established C‐terminal partner, Nup107, is preserved, while no interaction was detected between Nup133‐Cterm and mCenp‐F‐SID, either WT or R2687E. This observation is consistent with our previous studies using human Nup133 constructs that indicated that the C‐terminal domain of Nup133 does not interact with Cenp‐F
13.