Abstract
Neocortical pyramidal cells can integrate two classes of input separately and use one to modulate response to the other. Their tuft dendrites are electrotonically separated from basal dendrites and soma by the apical dendrite, and apical hyperpolarization-activated currents (Ih) further isolate subthreshold integration of tuft inputs. When apical depolarization exceeds a threshold, however, it can enhance response to the basal inputs that specify the cell’s selective sensitivity. This process is referred to as apical amplification (AA). We review evidence suggesting that, by regulating Ih in the apical compartments, adrenergic arousal controls the coupling between apical and somatic integration zones thus modifying cognitive capabilities closely associated with consciousness. Evidence relating AA to schizophrenia, sleep, and anesthesia is reviewed, and we assess theories that emphasize the relevance of AA to consciousness. Implications for theories of neocortical computation that emphasize context-sensitive modulation are summarized. We conclude that the findings concerning AA and its regulation by arousal offer a new perspective on states of consciousness, the function and evolution of neocortex, and psychopathology. Many issues worthy of closer examination arise.
Keywords: apical amplification, arousal, conscious state, context-sensitive modulation, hyperpolarization-activated currents, schizophrenia
Introduction
Recent evidence indicates that inputs to the apical synapses of mature neocortical pyramidal cells can modulate their response to basal inputs (Larkum, 2013), and it has been hypothesized that this is closely related to consciousness (Bachmann and Hudetz, 2014: Bachmann, 2015; and Meyer, 2015). Here, we first review the physiological evidence for modulatory effects of apical input, making clear what neurocomputational primitives we infer from it. Then, as adrenergic arousal is involved in regulating conscious state, we provide an in-depth review of its effects on apical function. We conclude that apical function, arousal, and conscious state are indeed closely related, but do not identify apical function with consciousness in general. As do many others (Koch, 2004), we take a pragmatic empirical approach to conceptions of consciousness, assuming that states in which we are awake or dreaming differ fundamentally from those in dreamless sleep or when under general anesthesia. We also assume that dream states differ in several ways from waking states, including greatly reduced levels of adrenergic activity, coherence, and cognitive control. They do have content, however, and the distinction between the level or state of consciousness and its contents is crucial. Bachmann and Hudetz (2014) argue that, in neocortex, apical inputs to pyramidal cells regulate the level of consciousness, whereas basal inputs specify the contents of consciousness. Our focus is on state rather than contents. One well-known way to quantify level and content is offered by information integration theory (Oizumi et al., 2014). After reviewing the evidence on apical function, we argue that it supports a similar theory (Kay et al., 1998; Kay and Phillips, 2011) that has recently been strengthened using advances in the formal analysis of Shannon information (Wibral et al., 2015). Thus, the central concerns of this review are with the effects of apical input on conscious state when awake, their regulation by both tonic and phasic changes in adrenergic arousal, and their relevance to anesthesia, to altered states of consciousness in dreams and psychosis, and to theories of neocortical function.
Given these concerns, we review three large bodies of research between which there has so far been little interaction even though all three are concerned with context-sensitive processes that are widespread throughout the neocortex. The first shows that, in addition to the somatic integration zone that generates action potentials (APs), some common classes of mature pyramidal cell have a functionally distinct zone near the top of the apical dendrite that integrates input to the apical tuft (Larkum, 2013). When this apical depolarization exceeds a threshold, it amplifies the cell’s response to the basal inputs that specify its selective sensitivity. This process is referred to as apical amplification (AA) (Larkum and Phillips, 2015; Muckli et al., 2015; Phillips et al., 2015). It selectively enhances those signals that are currently relevant in the context of activity elsewhere. Thus this may provide an intracellular mechanism for effects of context that are central to Gestalt organization, contextual disambiguation, priming, attention, cognitive control, and learning (Phillips, 2015). Much of the evidence for AA comes from in vitro studies. Some things are likely to be the same in vivo as in vitro, such as the morphological differences between apical and basal dendritic trees that are central to AA, but other things are likely to differ, such as the adrenergic regulation of communication between apical and basal zones. Hypotheses based on in vitro evidence therefore require testing in vivo, and we cite several such tests.
The second body of research is that concerned with the effects of both tonic and phasic arousal on context-sensitive modulation. This evidence clearly indicates that arousal enhances context-sensitivity such that high-priority signals are perceived and remembered even better while signals of lower priority are suppressed even more (Aston-Jones, 1985; Mather et al., 2015). The adrenergic system plays a central role in this via arousal-induced norepinephrine (NE) release from afferents of the locus coeruleus (LC), which is the pontine nucleus of the noradrenergic arousal system that innervates all of neocortex. An influential theory of the interactions between adrenergic arousal and selectivity (Aston-Jones, 1985; Aston-Jones and Cohen, 2004) has successfully generated much further research on these issues (e.g. Eldar et al., 2013). Studies of the effects of arousal on perception and learning have forged strong links between research on basic cognitive processes and research on emotional arousal (Mather et al., 2015). Though this research on arousal and that on AA have not yet been explicitly related, it has been suggested that one crucial function of arousal may be to enhance AA (Larkum and Phillips, 2015; Mather et al., 2015).
The third body of research is that concerned with the functions and mechanisms of consciousness. Our primary concerns within this large, diverse, and rapidly growing area are on the effects of arousal and on theories that explicitly relate consciousness to AA (Bachmann and Hudetz, 2014; Meyer, 2015). Sustained levels of adrenergic arousal have long been known to be related to states of consciousness as they are at a minimum during REM sleep, low during non-REM sleep, and much higher during wakefulness (Foote et al., 1983; Rajkowski et al., 2004). When awake, temporary phasic adrenergic bursts increase the focus of conscious processing on selected activities in a way that enhances perceptual awareness of and memory for those activities (Mather et al., 2015). Thus, evidence that AA depends upon noradrenergic arousal suggests that it may be involved in the regulation of conscious state. Furthermore, much of the input to apical synapses comes from the feedback, “top-down,” or recurrent connections that have long been considered to play a major role in conscious states (e.g. Cauller and Kulics, 1991; Lamme and Roelfsema, 2000; Koch et al., 2016). Relations between AA and conscious state therefore merit close examination.
Assessment of relations between arousal, AA, and conscious state requires us to consider two recent discoveries in neuroscience that will be unfamiliar to many readers. The first is that of AA itself. The second is the discovery that the communication between apical and somatic zones on which AA depends is regulated by arousal via nonsynaptic hyperpolarization-activated HCN-channels. For an in-depth review of HCN-channels see Biel et al. (2009). From these recent discoveries we infer that, in addition to the well-established primitives of excitation, inhibition, and disinhibition, three new primitives have evolved within neocortex thus extending its cognitive capabilities in fundamental ways. We review these recent advances in depth because they offer new perspectives on relations between mind, brain, and consciousness.
The HCN-channels that pass hyperpolarization-activated currents (Ih) have a high density in the apical tuft dendrites of L5 neocortical pyramidal neurons (e.g. Lörincz et al., 2002). They are nonsynaptic cation conductances that are tonically active at rest and therefore act as a leak conductance. This has the consequence that the distal apical tuft compartment of adult neocortical pyramidal neurons is electrotonically isolated from the basal compartment and has the same input resistance as the much larger cell body (Zhu, 2000). Combined with active properties that can produce local dendritic spikes, this results in the key feature of large pyramidal neurons: their ability to process functionally different streams of input separately. Given this arrangement and its dependence on Ih-channels, it becomes clear that regulation of those channels will have important consequences for the computational capabilities of pyramidal neurons (e.g. Berger et al., 2001; Harnett et al., 2015). This suggests a clear link between arousal and AA because Ih-currents regulate AA (e.g. Berger et al., 2003), and adrenergic arousal regulates Ih and thus the interaction between somatic and apical integration zones (e.g. Barth et al., 2008; Carr et al., 2007; Robbins and Arnsten, 2009). Put simply the evidence to be reviewed suggests that arousal enhances AA and regulates conscious state by controlling flow through Ih-channels.
The following sections are organized as follows. We first summarize evidence that supra-threshold depolarization at the apical integration-zone amplifies response to basal input. We then summarize evidence that adrenergic arousal enhances the effects of prioritization. The central body of the paper reviews evidence that AA is regulated by Ih-currents which are themselves regulated by the adrenergic system, thus providing a means by which arousal can enhance AA. These processes are then related to disturbances of context-sensitivity and states of consciousness in schizophrenia that may be due to mutations in the DISC1 gene that lead to excessive Ih, and thus reduced AA. Implications of these findings for theories of adrenergic arousal, neocortical computation, and consciousness are then discussed. Finally, we note reservations that must be placed on the inference that arousal regulates AA via Ih-currents, and list a few of the many questions that arise.
Suprathreshold Depolarization of the Apical Integration Zone Amplifies Response to Basal and Perisomatic Inputs
Amplification of some signals and suppression of others is a crucial function of the neocortex, but how do local cortical circuits know what to amplify and what to suppress? As noted above, it has often been suggested that one of the functions of arousal and attention is to increase the “signal” to “noise” ratio, but distinguishing between “signal” and “noise” is far from easy. Information that is highly relevant in some brain regions or in some contexts may be irrelevant or even detrimental in other regions or other contexts. Neurobiological and psychological evidence from a wide range of mammals from rodents to humans shows that the selectivity required to distinguish between the signals to be enhanced and those to be suppressed is highly dynamic and depends upon current tasks, thoughts, and emotions as well as upon current exteroceptive and interoceptive inputs. Dynamic context-sensitive processes are therefore required to distinguish between “signal” and “noise.” Apical amplification provides an intracellular mechanism serving that requirement.
The particular signals to be amplified or suppressed must be specified by intracortical interactions because they require highly informative and locally specific interactions. We therefore review recent evidence for intracellular and microcircuit mechanisms by which signals are either amplified or suppressed within neocortex, prior to their further modulation by the noradrenergic system. Direct evidence for AA is provided by patch-clamping studies showing that inputs to the apical tufts of pyramidal cells are integrated separately from inputs to their basal dendrites before being used to modulate the cell’s response. Current models of neocortex, including models of noradrenergic effects such as that of Aston-Jones and Cohen (2005), typically assume that pyramidal cells can be adequately thought of as point processors that simply sum all of their excitatory and inhibitory inputs and then use that single integrated value to determine action potential output. In contrast to that assumption the evidence for AA clearly shows that some pyramidal cells have not one but two main sites of integration such that, when apical depolarization coincides with suprathreshold basal input, calcium spikes initiated by a site of integration near the top of the apical dendrite amplifies the cell’s response to its basal and perisomatic inputs (Larkum et al., 1999; Larkum et al., 2004; Larkum et al., 2007; Larkum et al., 2009; Ledergerber and Larkum, 2010; Larkum, 2013). The most studied mechanism by which AA is implemented in layer 5 cells is referred to as back-propagation activated calcium spike firing (BAC firing). Local integration within the basal and tuft dendrites depends upon the regenerative activation of NMDA receptors (NMDA-spikes). AA may be fully implemented by NMDA-spikes alone in supragranular neurons (Palmer et al., 2014), but even in infragranular neurons, NMDA-spikes have an important influence (Larkum et al., 2009). Essential properties of these mechanisms are illustrated in Fig. 1. Though much of the evidence reviewed concerns pyramidal cells in layer 5, broadly similar conclusions also apply to supragranular neurons (Lucy Palmer, personal communication). Inhibitory interneurons that specifically target apical dendrites in layer 1, such as Martinotti cells, produce disamplification. They suppress amplification without inhibiting action-potential output.
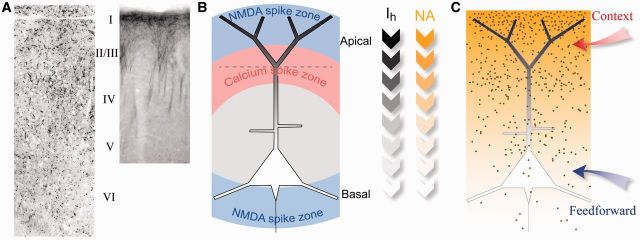
Figure 1.
Distribution of Ih and NE varicosities. (A) Left, Photomicrograph adapted from Audet et al. (1988) showing the distribution of noradrenergic varicosities (black dots) found throughout the neocortex. Right, immunocytochemical labeling of HCN (Ih) channels in the neocortex from Lörincz et al. (2001). (B) Schematic diagram of integration zones in L5 neocortical pyramidal neurons showing the areas of the dendrite that evoke Ca2+ and NMDA spikes (Larkum et al., 2009). The graded shading and arrows denote the density of Ih-channels (gray) and NE receptors (NA) (yellow in the online version) embedded in the dendritic membrane of L5 neurons. (C) Schematic diagram of the distribution of NE varicosities found throughout the neocortex (black dots) relative to the distribution of Ih in pyramidal neurons (shown by shading of the dendrites).
Evidence reviewed in detail by Phillips (2015) provides four distinct neurobiological grounds for asserting that excitatory apical input amplifies the cell’s response to its basal input. First, the findings of multisite intracellular patch-clamping studies directly show that apical input can lead to dendritic spikes that are initiated locally in the apical tuft dendrites. Second, the electronic distance of tuft input from the somatic zone of AP generation puts that input in a special position that differs from that of basal/perisomatic input. This difference increases with increases in the length of the apical dendrite. Third, the apical integration zone receives input from sources differing greatly from that of basal input. Tuft synapses in layer 1 receive long-range inputs that convey contextual information from diverse feedback, lateral, thalamic, and subcortical sources, whereas the basal and perisomatic inputs receive feed-forward synaptic inputs that specify the cell’s receptive-field selectivity. Fourth, computational studies show that neurons with functionally distinct apical and somatic zones of integration can provide the context-sensitivity shown by a wide variety of perceptual and cognitive functions. In addition to these four grounds high-resolution layer-specific fMRI has now shown that, in scene perception by humans, the most superficial layers are sensitive to contextual information from the surround but deeper layers are not (Muckli et al., 2015), providing further evidence that context-sensitive modulation is mediated by inputs to the tufts of pyramidal cells.
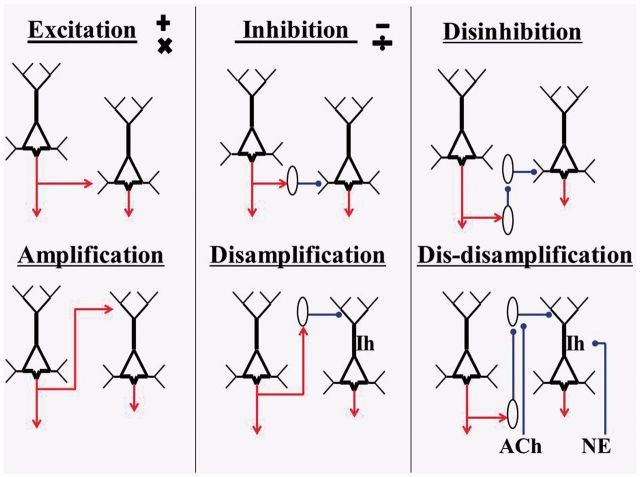
Overall, these findings suggest that apical amplification, together with the disamplifying effects of inhibitory interneurons that specifically target the tuft, provides neurocomputational primitives that can be clearly distinguished from the well-known primitives of excitation, inhibition, and disinhibition as shown by the simple abstractions in Fig. 2. This figure distinguishes six kinds of interaction between pyramidal cells. Amplification must be distinguished from somatic excitation because, in the absence of basal/perisomatic depolarization, depolarizing input to the tuft can be strong while having little or no effect on AP generation. Disamplification must be distinguished from basal/perisomatic inhibition because it can be strong without inhibiting AP output. Dis-disamplification must be distinguished from disinhibition because its effects are dependent upon the presence of excitatory input to the apical integration zone. These primitives can be combined into various circuits and local microcircuits. They provide motifs that recur throughout neocortex independently of whether or not they do so via a single canonical microcircuit. Whatever the nature of those microcircuits the evidence we review shows that AA is dependent on the current state of arousal. Changes in that state are so dynamic and context-sensitive that they underlie much of the trial-to-trial variability observed in neuronal response to sensory input (McGinley et al., 2015; Safaai et al., 2015). Relevant research on adrenergic arousal is therefore summarized in the following section before relating it to AA and Ih-currents.
Figure 2.
Primitive interactions from which neocortical circuits are built. The generic form of pyramidal cells is shown in a way that distinguishes their apical and basal trees. No attempt is made to show any columnar organization. The two cells shown in each of the six sections could be in either the same or different columns, or even in different cortical regions. They are shown at different heights in the diagram simply for diagrammatic convenience. Hyperpolarization-activated currents are shown as Ih. Inhibitory interneurons are shown as ovals. Cholinergic inputs are shown as ACh. Adrenergic inputs are shown as NE. Inputs from both of these subcortical modulatory systems tend to increase activation, and presumably in complementary ways. The outputs of individual neurons could combine these primitives in various ways. For example, the outputs of a given pyramidal cell could be excitatory at some of its projective sites and amplifying at others, or an inhibitory interneuron could combine disinhibition with dis-disamplification by inhibiting interneurons that target the soma as well as those that target the tuft.
Adrenergic Arousal Enhances the Effects of Prioritization
The effects of adrenergic arousal and its interaction with prioritization are many and varied [see Mather et al. (2015) for an in-depth review and expert commentary]. Aston-Jones (1985) proposed that a major function of adrenergic arousal is to improve the signal to noise ratio, and much evidence supports this. More recently, behavioral evidence from cognitive neuroscience, together with in vitro evidence revealing glutamatergic NMDA-receptor mediated modulation of NE release from cortical varicosities, has further strengthened the proposal that competing cortical representations are regulated by an interaction between glutamate and NE (GANE) (Mather et al., 2015). This proposed mechanism depends on postsynaptic receptors that have an essential role in spike-timing-dependent cortical plasticity (Huang et al., 2012) with one subtype being linked to long-term potentiation (LTP) and another to long-term depression (LTD). NE concentrations that mediate LTP effects are an order of magnitude higher than those that mediate LTD effects (Salgado et al., 2012). Thus, NE “hot spots” at synapses with high levels of glutamate release recruit postsynaptic receptor actions supporting short- and long-term strengthening of the most active circuits. Lateral inhibition and the effects of LTD on less active circuits additionally contribute to the winner-take-all and loser-take-less dynamics. The recruitment by priority of LC-NE hotspots through GANE explains a wide range of arousal-associated effects in cognition (Mather et al., 2015). This perspective implies that cortico-cortical interactions prioritize selected signals prior to their further enhancement by LC-NE input. As reviewed in detail below, AA could provide the locally specific intracortical prioritization on which such theories of arousal depend.
Ih-Currents Regulate AA
As depicted in Fig. 1, many studies show that the density of Ih-channels is high in the apical tree of pyramidal neurons (e.g. Williams and Stuart, 2000; Berger et al., 2001; Larkum et al., 2001; Lörincz et al., 2002, Kole et al., 2006), but low in other compartments (e.g. Acker and Antic, 2009; Nevian et al., 2007). This density increases greatly with distance from the soma along the apical trunk and plateaus at a uniformly high level within the tuft (Harnett et al., 2015). The Ih-channels that have the fast biophysical properties best suited to rapid computations are the hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels with HCN1 subunits, and it is Ih-channels with those subunits that are common in the apical tree of pyramidal neurons (Berger et al., 2001, 2003). Put simply, Ih-channels reduce the coupling between apical and somatic integration zones by providing a short-circuit that reduces current flow along the dendrite by increasing current flow across the dendritic membrane.
Dense Ih-currents increase the electrotonic distance between adjacent dendritic compartments, so they tend to normalize integration within the tuft (Magee, 1999) and reduce subthreshold communication between apical and somatic integration zones (Berger et al., 2001). This applies only at subthreshold levels of postsynaptic potential, however, because the activation curve for these channels is such that they inactivate with depolarization. This has the consequence that subthreshold events are processed almost exclusively in their respective compartments (apical or basal) until threshold is reached for a local spike that can then influence the other compartment. Another crucial consequence of tonic leak conductance in the tuft is that the resting membrane potential is shifted ∼10–20 mV unevenly as a function of location such that the tuft dendrite is tonically more depolarized than the cell body under control conditions. On the other hand, the local input resistance is constant over much of the dendritic membrane well into the tuft dendrite (Zhu, 2000). The average effect of blocking Ih-channels on tuft resting membrane potential is to hyperpolarize by 17 mV, whereas the same manipulation causes a shift of only 11 mV at the soma (Berger et al., 2001) rendering the resulting membrane potential everywhere in the cell the same without Ih-current. The corollary is that, counterintuitively, blocking Ih actually decreases excitability at proximal locations because the proximal resting potential is more hyperpolarized without the compensatory influence of local dendritic regenerative currents that occur in the tuft dendrite (Harnett et al., 2015). Blocking Ih activity also increases temporal integration within tuft dendrites and enhances the effects of apical depolarization on AP generation (Berger et al., 2001, 2003; Larkum et al., 2009; Harnett et al., 2015). In summary, the high density of Ih in the tuft enhances the contribution of local spikes (Ca2+ and NMDA) to the firing of the neuron and reduces the influence of subthreshold synaptic input over large distances. In other words, Ih provides an efficient biophysical mechanism by which pyramidal cells can function as a two-compartment neuron with two sites of integration and with a dedicated, threshold-dependent, form of communication between them.
The high density of Ih-channels in the distal apical trunks and tufts is not present at early stages of neocortical development. In rats, their density in the distal apical trunk and tuft increases 10-fold during the early stages of development (Atkinson and Williams, 2009). Combined with the emergence of robust Ca2+ spikes in the tuft region (Zhu, 2000), this means that in young animals (<post-natal day10 in rodents) pyramidal neurons act as compact single-site integrators, and develop into integrators with distinct sites for somatic integration and apical modulation as the neocortex grows in size and the density of synapses in the tuft increases. In the distal apical dendrites of mature layer 5B neurons there is also a high density of KV channels, suggesting that Ih and KV conductances could interact to control the initiation of dendritic spikes (Harnett et al., 2013, 2015). Blocking KV channels enhances the effect of tuft input on AP generation, but has no effect on AP generation by basal inputs alone (Harnett et al., 2013).
Given that Ih occurs throughout neocortex we need to understand its functions, and several possibilities have been suggested. There is evidence that in region CA1 of the hippocampus Ih-currents can normalize temporal summation so that it becomes independent of apical distance (Magee, 1999). We know of no evidence that AA occurs in three-layer structures such as hippocampus and olfactory cortex, however, so our working hypotheses is that the far greater evolutionary expansion of six-layer neocortex rather than of the more ancient three-layer cortex is in part due to the enhanced capabilities consequent upon the emergence of AA in neocortex. There is a little evidence that normalization by Ih may also occur in the neocortex (Williams and Stuart, 2000; Berger et al., 2001), but other functions for Ih are more likely there. First, they may enhance the temporal precision of coincidence detection by shortening the duration of temporal integration (Berger and Lüscher, 2003). Second, there is evidence that they constrain synaptic plasticity at distal apical dendrites (Nolan et al., 2004). Third, they may linearize integration within the tuft because of their uniformly high density there (Berger et al., 2001; Harnett et al., 2015). Fourth, they enhance suprathreshold interactions between apical and somatic zones (Berger et al., 2001, 2003), while suppressing subthreshold interactions so that basal inputs do not trigger apical calcium spikes in the absence of tuft depolarization (Berger et al., 2003). Fifth, regulation of both tonic and phasic states of arousal by NE may involve its suppressive effects on Ih-currents (Robbins and Arnsten, 2009).
Each of the five possible functions for Ih-currents listed above is likely to be of importance at some stage of development in some regions of some species. There is plenty of room for further studies of each of these possible functions, but it is clear now that in mature neocortex Ih-currents isolate the subthreshold integration of synaptic inputs to their respective subcellular compartments and regulate the generation of local spikes that communicate between apical and somatic compartments. This all suggests that the contribution of the apical zone to the generation of action potentials depends upon the regulation of Ih-currents.
Regulation of Ih-currents is not a simple matter of having them fully open or fully closed, however. Both extremes are likely to be malfunctional. If the Ih-currents are too strong then there will be little or no communication between the apical and somatic zones. This could lead to incoherent and unregulated responses to sensory input, and thus to high levels of distractibility and uncontrolled impulsive responses. In cases where driving input is internally generated this could lead to thoughts that combine incoherent elements and which are inadequately related to the evolving train of thought. For all these reasons we assume that Ih-currents can be too strong.
We also assume that Ih-currents can be too weak. A severe reduction of Ih by the inherited absence of HCN1 channels can produce pathologically strong epileptic AP output (Kole et al., 2007). Even when less severe, weaknesses in Ih can still be malfunctional because AP output could then be generated by either basal or apical input alone. Consider first the case where APs are generated by apical depolarization alone. This is likely to lead to incoherent unpredictable outcomes because the cell’s output will then lose its dependence on the basal input that specifies the cell’s selective sensitivity. Now consider the case where APs are generated by somatic depolarization alone. Calcium spikes from the apical integration zone could then be triggered by back-propagating APs alone, and that would further increase AP output whatever the tuft input. This effect could be of adaptive advantage in producing fast strong feedforward activity in highly arousing situations that trigger rapid “reactive” responses. It would not be adaptive in situations where slower more “reflective” processing is the better option, however. This may be important in many social situations where better outcomes are achieved by reflective than by impulsive reactions (Strack and Deutsch, 2004). A key property of AA is that it facilitates a form of recurrent reflective processing in which a wide range of neocortical activities can influence each local processor so as to produce coherent patterns of overall activity. This is a process known as “relaxation” in neural-network theory (Amit, 1989). Relaxation takes time, however, and there are situations in which rapid responses are required. In those cases, it may be useful for AP generation by feedforward basal drive to take precedence over relaxation by iterations of AA, and this could be achieved by reducing Ih-currents, which is exactly the effect produced by high levels of NE during stressful situations. Ih-levels must therefore be controlled so that they are both well suited to current needs and neither too strong nor too weak. The following section therefore reviews evidence that prefrontal and neuromodulatory systems are involved in setting those levels.
Arousal Regulates Ih-Currents
Ascending influences from subcortical noradrenergic and cholinergic systems interact to regulate the firing mode of layer 5 pyramidal cells by modulating both voltage-dependent and voltage-independent conductances (Wang and McCormick, 1993) as indicated in Fig. 2. These subcortical systems are themselves regulated by descending influences from neocortex. Signals from the LC regulate the overall level of tonic background adrenergic activity and can also produce temporary phasic increases in adrenergic activity (Aston-Jones and Cohen, 2004; McGinley et al., 2015; Mather et al., 2015). NE receptors are well placed to regulate Ih-currents because, as shown in Fig. 1, their subcellular distributions are closely matched. Adrenergic activity is known to play a crucial role in the working memory functions of primate prefrontal cortex (PFC) and to do so by modulating Ih-currents (Li and Mei, 1994; Wang et al., 2007). For a recent review see Robbins and Arnsten (2009). These currents weaken the effects of nearby synaptic inputs, and α2a-adrenoreceptors are well positioned to modulate their activity (Wang et al., 2007). Pharmacological, electrophysiological, and genetic knockdown studies have all been interpreted as indicating that when activated in dorsolateral PFC these adrenoreceptors weaken nearby Ih-currents (Li et al., 1999; Ramos et al., 2005; Wang et al., 2007).
In some regions of some species, the effects of adrenergic activity on Ih-currents may be mediated by modulation of Ca + 2-cAMP signaling (Arnsten et al., 2012). Arnsten et al. argue that NE suppresses Ih-currents in primate dlPFC by reducing cAMP levels in spine necks via α2a-adrenoreceptors. This argument is based on three grounds. First, α2a-adrenoreceptors colocalize with Ih-currents near the synapse and in the spine neck. Second, stimulation of α2a-adrenoreceptors, with an α2a-adrenoreceptor agonist increases pyramidal cell response to those inputs to which it is selectively sensitive (Wang et al., 2007). Third, blockade of α2a-adrenoreceptors with an antagonist causes a collapse of dlPFC network firing (Li et al., 1999) that can be restored by blocking Ih-channels (Wang et al., 2007). A major goal for future research will be to determine to what extent these intracellular processes depend upon species, region, and maturity.
Arousal Enhances AA by Reducing Apical Ih-Currents
Given evidence that adrenergic arousal reduces Ih-currents and that Ih-currents reduce AA we infer that adrenergic arousal enhances AA by reducing Ih-currents. This inference has been directly confirmed in the case of rat PFC. There it has been shown both that NE enhances the generation of apical calcium spikes by back-propagating APs and that this is done by inhibiting Ih-currents (Barth et al., 2008). Voltage-clamp analysis in slices of rat PFC also found that α2-NE receptor stimulation inhibited Ih and enhanced temporal summation during trains of distally evoked EPSPs (Carr et al., 2007). The net effect of adrenergic stimulation was to suppress response to isolated excitatory inputs while enhancing the response to a coherent burst of synaptic activity. Further support for this view is provided by evidence that α2a-adrenoreceptors are located on the apical dendrites of PFC pyramidal neurons in monkeys (Aoki et al., 1998).
Though subject to reservations noted in the final section, it seems likely that by opposing Ih-currents both tonic and phasic adrenergic arousal will increase the contribution of the apical integration zone to AP-generation. Thus, interactions between adrenergic arousal and AA may help us understand how widespread changes in conscious state affect fundamental processes of perception and cognition. Further evidence for this comes from studies of genetic and cognitive impairments in schizophrenia, as reviewed in the following section.
Genetic and Cognitive Impairments in Schizophrenia may Involve AA
Schizophrenia has been associated with translocations of the disrupted in schizophrenia 1 (DISC1) gene (St Clair et al., 1990; Millar et al., 2000; Blackwood et al., 2001; Robbins and Arnsten, 2009). This gene is related to cognitive functioning and to structural PFC alterations in the general population (Chubb et al., 2008). It is known to regulate cortical development in animals (Hikida et al., 2007), and its abnormal expression has been related to abnormal neurodevelopment in schizophrenia (Cannon et al., 2005). For example, two groups (Hikida et al., 2007; Pletnikov et al., 2008) studied the effects, in mice, of human DISC1 cDNA transgenes designed to express a truncated DISC1 protein, similar to that associated with schizophrenia. Both studies found increased lateral ventricle size, as measured using MRI, a feature that has often been found in both people with schizophrenia and in their unaffected first-degree relatives.
DISC1 may affect Ih, and thus AA, via its effects on cAMP signaling. The protein produced by the DISC1 gene binds to and regulates phosphodiesterase-4 isozymes (PDE4s), which normally increase hydrolysis of cAMP under conditions of high cAMP concentration (Millar et al., 2007; Millar et al., 2005). The reduction in DISC1 in schizophrenia caused by the translocation could therefore lead to excessive cAMP levels, which then leads to excessive Ih (Wang et al., 2007). Indeed, mice with a DISC1 alteration that is homologous to the human DISC1 translocation show decreased PDE4 activity and excessive cAMP levels (Millar et al., 2005; Kvajo et al., 2011), which lead to increased Ih and reduced AA. Moreover, mouse models of the DISC1 translocation in schizophrenia produces animals with cognitive impairments that resemble those found in schizophrenia (Koike et al., 2006; Kvajo et al., 2008).
The cognitive impairments in schizophrenia are not limited to functions of the PFC (Paspalas et al., 2013), or hippocampus (Kim et al., 2012; Inta et al., 2014), and include many that could be due to a reduction in AA-dependent context-sensitivity. It has been clearly shown that schizophrenia is characterized by a widespread impairment in cognitive coordination, reflecting a disturbance in the basic processes responsible for the adaptive use of contextual information to guide the processing and learning of feedforward input (Phillips et al., 2015; Phillips and Silverstein, 2003, 2013). This disturbance, which is manifested in many perceptual, attentional, memory, linguistic, and learning changes (Phillips and Silverstein, 2003, 2013; Phillips et al., 2015), could arise from disturbances in AA (Phillips, 2015), consistent with computational arguments and network simulations based on information theory (Phillips and Singer, 1997; Kay and Phillips, 2011; Silverstein et al., submitted). Though much of the past work on the role of cAMP-Ih signaling in regulating dynamic changes in the strength of functional connections has focused on the PFC (Arnsten, 2009; Vijayraghavan et al., 2007; Paspalas et al., 2013), impairments of context-sensitivity in schizophrenia also occur in many other neocortical regions (Phillips and Silverstein, 2003; Silverstein et al., 2009). DISC1 translocation-related excessive cAMP-Ih signaling and thus reduced AA could account for many forms of the reduced influence of context that are observed in schizophrenia (Phillips et al., 2015). Our previous emphasis on NMDA-receptor malfunctions in schizophrenia (e.g. Phillips and Silverstein, 2003) remains valid because AA depends on normal NMDA-receptor activation (Major et al., 2013; Palmer et al., 2014). Finally, schizophrenia patients with high levels of orexin A, a neuropeptide that regulates arousal level, have lower levels of negative and disorganized symptoms, the symptoms most associated with cognitive impairment in this disorder (Keri et al., 2005; Torniainen et al., 2012; Minor et al., 2015). They also have better cognitive functioning compared to patients with low levels of orexin A (Chien et al., 2015). This suggests that arousal, as long as it is kept within tolerable limits (Vijayraghavan et al., 2007; Hains and Arnsten, 2008; Hains et al., 2015), could be a cognition enhancing mechanism for schizophrenia. This hypothesis is consistent with much work on the positive effects of nicotine on cognition in patients with the disorder who smoke (Harris et al., 2004), on arousal level in nonpatient smokers (Kalman and Smith, 2005), and on the effects of smoking on NE release (Sterley et al., 2014).
Prima facie it might seem that psychotic delusions could be due to excessive effects of internally generated or “top-down” activity mediated via the tuft synapses in layer 1. If that were the case, however, then such psychotic states would be associated with Ih-currents that are too weak. This contrasts with the evidence on DISC1, which suggests that Ih-currents are too strong. Strong Ih opposes the effects of tuft input on AP output, indicating that the content of psychotic thoughts is conveyed predominantly by the basal synapses, with little or no modulation by apical input. This suggests that such thoughts are psychotic, not because they have an internal origin, but because they lack the coherence and sensitivity to current circumstances normally imposed by the contextual modulation that is mediated by apical input.
Implications for the GANE Theory of Adrenergic Arousal
The evidence for noradrenergic control of Ih-currents suggests a major expansion of the GANE model that was proposed by Mather et al. (2015). The high density of LC noradrenergic varicosities in layers 1 and 2 of neocortex (Audet et al., 1988; Agster et al., 2013) is consistent with a special role of apical tufts in mediating the prioritizing function of cortico-cortical connections required in GANE. For example, tonic arousal associated with LC-NE neuronal activation (Carter et al., 2010) sets up an “alert” state for environmental monitoring (Constantinople and Bruno, 2011; Polack et al., 2013). Increased NE levels will increase the effects of tuft input by suppressing the Ih-currents that isolate the tuft, thus enhancing the selective amplification of contextually appropriate signals. When changing contingencies occur, phasic NE release (Bouret and Sara, 2005), and GANE mechanisms would be recruited. Under the GANE model, the glutamate amplification of phasic NE release occurs when varicosities are maximally depolarized and glutamate release is sufficient to engage NMDA receptors through spillover. For evidence that this increases local NE concentrations to a level that engages selective mechanisms for resource allocation and the promotion of plasticity see Mather et al. (2015). AA provides a selective mechanism with the required local specificity. It strengthens circuits with co-incident apical and basal dendritic depolarizations thus leading to flexible context-sensitive behaviors in both the short-term and the long-term. Finally, it must also be noted that, although the GANE theory is to a large extent supported and extended by the studies reviewed above and by the neurocomputational theory of AA discussed next, neither theory logically entails the other.
Implications for the Coherent Infomax Theory of Neocortical Computation
These studies of arousal and Ih-currents make important contributions to our understanding of the computational capabilities of neocortical pyramidal cells. Four distinct neurobiological grounds were given above for the view that input to the tuft of pyramidal cells enhances their capabilities by amplifying their response to basal/perisomatic input. Studies of Ih-channels add to this view in three ways. First, their high density in the apical tree but low density in the basal/perisomatic tree provides direct anatomical grounds for distinguishing between apical and basal/perisomatic input. Second, those studies provide evidence that within the main trunk of the apical dendrite active currents do not compensate for electrotonic distance so as to approximate a “dendritic democracy” (Branco and Häusser, 2011). On the contrary, they increase the functional separation between the apical and somatic integration zones. Third, those studies suggest that Ih-currents provide a means by which subthreshold integration in the apical zone can be isolated from the somatic zone while facilitating suprathreshold interactions such that apical depolarization turns single APs into a burst of 2–4 APs lasting about 10–20 ms (Larkum, 2013).
All these findings provide some support for theories of neocortical computation that emphasize context-sensitive modulation, such as those of counter-stream architectures (Ullman, 1995) and free energy reduction (Friston, 2010). They provide particularly strong support for the theory of coherent infomax (Kay and Phillips, 2011; Wibral et al., 2015), however, because the capabilities that AA and its regulatory mechanisms provide are exactly those required by that theory (Phillips, 2015). The theory of coherent infomax has been rigorously formulated in information theoretic terms and, in addition to correctly predicting the activation function now confirmed by the findings related to AA, it includes a biologically plausible learning rule derived analytically from the proposed information-processing objective (Phillips et al., 1995; Kay, 1999; Kay and Phillips, 2011, Wibral et al., 2015). It has been tested by large computational simulations (Kay et al., 1998), and has been related in detail to a wide range of empirical findings from several disciplines (Phillips, 2015). In short, the theory of coherent infomax hypothesizes that local cortical processors can amplify relevant activities and suppress irrelevant activities because they receive both receptive field inputs that specify the semantic content of their outputs and contextual field inputs that inform them of activity elsewhere in the system. This enables them to search for holistic Gestalt patterns of activity that maximize coherence across the system as a whole. Though that theory was not originally committed to the hypothesis that the capabilities that it requires are implemented at a cellular level, it did explicitly predict that local processors with exactly those capabilities are replicated many times over across the neocortex. That prediction has now been confirmed in a way that clearly implicates the intracellular processes of AA (Phillips, 2015).
The theory of coherent infomax has much in common with that of integrated information theory (Oizumi et al., 2014), but differs in incorporating recent advances in the formulation of Shannon information (Wibral et al., 2015), and in emphasizing AA as a way in which coherent infomax could be implemented in neocortex. Coherent infomax as implemented by AA offers a perspective on relations between arousal and cognition that advances beyond previous conceptions (e.g. Aston-Jones and Cohen, 2005). It builds on the evidence that neocortical pyramidal cells are not simply integrate-and-fire point processors but have two functionally distinct sites of integration, and uses that to explain how context-sensitive modulation can be locally specific in space and time. Put simply, from the perspective of this theory, modulation of cortical activity by subcortical systems is seen as enhancing the modulatory interactions that occur within the neocortical system itself.
Implications for Theories Relating Apical Amplification to States of Consciousness during Wakefulness, Sleep, and Anesthesia
Bachmann and Hudetz (2014) and Meyer (2015) review evidence relating states of consciousness to the amplifying effects of input to the apical tufts of pyramidal cells in neocortex. The conclusion inferred from this evidence by Bachmann and Hudetz (2014) is that basal inputs specify the contents of consciousness and apical inputs determine the level of consciousness. They argue that basal input alone generates short-lived EPSPs and fragmented cortical neural activity but not a sustained “field of consciousness.” If there is also sufficient modulatory input to the apical dendrites of the cells conveying the cognitive content, however, then this amplifies their neuronal output, thus producing the integrative computation experienced as the normal, context-sensitive, state of wakeful consciousness. Bachmann and Hudetz (2014) present several arguments in support of their theory, and draw on empirical evidence from various disciplines, including psychophysical studies of the time-course of visual masking, and studies of the effects of anesthetics on dendritic calcium spikes and on the N1 event-related potential that is associated with awareness. Meyer (2015) reviews evidence concerning the cellular mechanisms involved in the effects of a wide variety of general anesthetic agents. He concludes that most of them operate by interfering with the modulatory effects of apical input. This includes a link to Ih-currents because a subset of general anesthetics that includes isoflorane, halothane, propofol, and ketamine has been shown to produce tonic suppression of Ih-currents (Meyer 2015; Zhou 2012). Meyer (2015) argues that when Ih is fully blocked, somatic activation alone may trigger a dendritic calcium spike, leading to a breakdown of the mechanism by which only contextually prioritized signals are raised above the level of general background activity.
Studies of apical activity in awake rodents provide evidence that AA might be involved in cognitive behavior (Murayama et al., 2009; Xu et al., 2012; Cichon and Gan, 2015; Manita et al., 2015). In studies of human perception, overt detection of near-threshold stimuli has often been interpreted as an indicator of perceptual awareness, with processing below the level of detection being described as subliminal. Testing whether perceptual awareness of near-threshold stimuli depends upon AA is therefore a critical step toward establishing the importance of AA for conscious perception. The AA hypothesis predicts that (i) apical activity should be positively correlated with perceptual detection; and (ii) suppressing it should impair detection. These predictions have recently been tested in rodents using two-photon imaging of apical Ca2 + activity in layer 5 pyramidal neurons, and preliminary data suggest that these predictions are indeed correct (Takahashi and Larkum, 2016).
The altered state of consciousness experienced as dreaming may show what happens when activity that is initiated internally is transmitted through the cortical system via basal and perisomatic synapses in the absence of the contextual modulation that would help make it relevant and coherent when awake. We infer that unmodulated transmission of cognitive content occurs during dreams because, although cholinergic activity increases greatly during REM sleep, NE activity is switched off, thus maximizing Ih and isolating apical input from the somatic zone that generates action potentials. This reminds us that the feedforward drive transmitted via basal synapses cannot in general be identified with activity of external origin. It may also help explain why dreams, and other examples of what Freud called primary process thinking, seem to share the property of bizarre incoherence with psychosis, as noted long ago (Jung, 1907). Experimental studies (Scarone et al., 2008) provide quantitative evidence of this similarity between dreaming and psychotic states, and it can be interpreted as indicating that AA-dependent contextual modulation is minimal or absent in dreaming and weakened in psychosis.
Grounds for caution when relating consciousness to AA have been noted in a previous paper (Phillips, 2015) and responded to Bachmann (2015), but those discussions did not consider the evidence on arousal, Ih, and AA reviewed above. Overall, this evidence supports and extends theories relating AA to conscious state because it shows that AA depends upon levels of adrenergic arousal that vary from being off or low during REM and slow wave sleep, moderate during waking with high phasic activity to relevant stimuli, and with excessive tonic activity when stress is too high (Foote et al., 1983; Rajkowski et al., 2004; Mather et al., 2015). This suggests that AA is at a minimum during dreaming and that waking conscious states depend upon both tonic and phasic levels of AA.
Relating consciousness to AA may shed light on functions of consciousness because all of the cognitive functions that have been explicitly related to AA (as reviewed by Phillips, 2015) are either likely to require some form of consciousness (e.g. selective attention, WM, and cognitive control) or are enhanced when conscious (such as learning, Gestalt organization, and contextual disambiguation). Our learning abilities, for example, are greater when we are conscious than when we are not. There are strong grounds for relating learning in neocortex to AA because that produces large calcium currents, which play a leading role in synaptic plasticity (Sjöström et al., 2008; Chichon and Gan, 2015). Therefore, the speed with which dreams are usually forgotten could be due to the exceptionally low levels of adrenergic activity, and thus of AA, during dreaming. Gestalt organization is also enhanced when conscious. Though some forms of Gestalt grouping occur pre-attentively, others require attention (Phillips et al., 2010), and thus some form of consciousness. Much evidence suggests that states of focused attention when awake can be seen as states in which the tonic changes in adrenergic arousal that occur on waking are briefly enhanced so that they become more locally specific in space and time (Harris and Thiel, 2011; McGinley et al., 2015). Given these considerations, our working hypothesis is that apical amplification, disamplification, and dis-disamplification enable or enhance cognitive capabilities such as those listed above, and that, via the adrenergic system, they are turned on when we awake and depend upon arousal level when awake.
Grounds for caution when relating consciousness to AA still remain, however, and we do not identify AA with consciousness in general. First, AA and its regulation by adrenergic arousal are concerned with variations in conscious state, and it has been argued that an adequate understanding of consciousness requires state-based approaches to be combined with those concerned with content (Hohwy, 2009). The evidence and arguments presented here strengthen the distinction between state and content, but they are not primarily concerned with mechanisms by which the various contents of consciousness are differentiated from each other. Second, Ih is maximal during REM sleep because adrenergic activity ceases. This minimizes AA but does not prevent dreaming. Dreams clearly have semantic content so any phenomenal consciousness associated with them does not require levels of AA associated with waking. Furthermore, although dream states can be characterized as flow of activity that is low on coherence because it is unconstrained by AA, that does not explain why those states occur. Third, the evidence reviewed strongly suggests that the capability for AA within pyramidal cells arises late in the course of both phylogenetic and ontogenetic development. We know of no evidence that it occurs in hippocampus, olfactory cortex, or subcortical regions. So, unless we deny any form of consciousness to infants or to species lacking a neocortex, we must distinguish between different forms of consciousness across individuals and species as well as to variations over time within individuals. Fourth, the evidence indicates that AA has less effect on fast reactive feedforward processing than on slow reflective processing. Although states dominated by reactive processing differ from those dominated by reflective processing we do not consider them to be unconscious. Finally, although AA may be an especially effective way of maximizing coherent or integrated information it is not the only way, so other ways may also be associated with some form of consciousness. In response to these reservations it could be argued that (i) consciousness is not all or none but occurs in various states; (ii) dreams are a distinct state of consciousness; (iii) waking conscious states of mature neocortex depend upon AA; (iv) awake states vary in the selectivity of attention and in the degree to which they are reactive or reflective. Prima facie, such arguments do not seem implausible, but their validity remains to be determined.
Reservations and Issues Arising
Four major reservations must be placed on the inference that regulation of Ih-currents provides a route by which arousal can enhance AA. First, it is only in a few regions of a few species and at late stages of ontogenetic development that AA and arousal-dependent regulation of Ih-currents have been shown to occur. Uncertainties concerning the extent of their adaptive radiation and evolutionary improvement demarcate a largely unknown territory that remains to be explored. We assume that there is no single optimal solution to the problem of distinguishing between relevant and irrelevant signals, so there is plenty of room for evolutionary advances to improve on the strategies for doing so. There are good grounds for assuming that there is substantial heterogeneity across regions and species in these intracellular processes (Arnsten et al., 2012), but we are only at the early stages of mapping that heterogeneity. It may well be that advanced forms of AA will be found in primate and human PFC, but whether that is so or not remains to be seen. Second, though it has been directly demonstrated that NE can regulate AA by inhibiting Ih-currents (Carr et al., 2007; Barth et al., 2008), such demonstrations are rare and far more are required. Furthermore, explanations of the effects of NE on neuronal activity in PFC have been proposed that make no use of apical inputs (e.g. Arnsten, 2012). Thus, by themselves, those particular effects in PFC do not provide conclusive evidence on either AA or its regulation. If the enhancement of AA by NE observed by Barth et al. (2008) and Carr et al. (2007) is rare, or confined only to a few regions, then that would provide a strong constraint on the inferences that we have drawn. In contrast to that possibility, however, those findings suggest that, if adequate levels of NE are ensured, then effects of AA could be observed in cases where they have so far seemed weak or absent, as at early stages of ontogenetic development, for example. Third, even where it has been directly demonstrated that arousal enhances AA by reducing Ih-currents that does not demonstrate that no other routes for such enhancement exist, nor does it demonstrate that no routes exist by which arousal can impair performance by suppressing AA. It has often been shown that, although intermediate levels of arousal can enhance performance, even higher levels that activate low-affinity adrenoreceptors can be detrimental to several cognitive functions (e.g. Robbins and Arnsten, 2009; Mather et al., 2015). It seems likely that this will also apply to AA, but the extent to which it does so remains to be determined. Fourth, new information theory measures have been developed by which the contributions to output of two functionally distinct sources of input can be assessed quantitatively (Wibral et al., 2015). Those measures can definitively distinguish modulatory from driving effects so they could be applied to multisite patch-clamp data that distinguishes basal input, tuft input, and AP output. This has not yet been done, however. When it is it will provide a direct formal test of the inference that apical input is modulatory with its effects being enhanced by NE.
Contextual guidance of learning and processing by modulatory interactions that do not corrupt signal semantics is central to the theory of coherent infomax, but such a capability is also incorporated, either explicitly or implicitly, in several other neurocomputational theories (e.g. Ullman, 1995). A major goal for future research will therefore be to relate the evidence on AA and arousal to those theories. As an early step toward that long-term goal we note that the well-known theory of free energy reduction, predictive coding, and the Bayesian brain (Friston, 2010), has been expanded to include an account of the experience of conscious presence and self-hood (Seth et al., 2011; Seth, 2013). This theory has not yet been explicitly related to the evidence for AA and its regulation by arousal, though it has much in common with the theory of coherent infomax, including a prominent role for modulatory feedback and lateral connections (Phillips, 2015, Section 4.3; Phillips et al., 2015, Section 6). Put simply, predictive coding theories emphasize reducing prediction error, whereas coherent infomax emphasizes increasing prediction success, thus implying both similarities and differences between the two theories. Prima facie, the evidence for AA and its regulation by arousal provides stronger support for coherent infomax because that theory emphasizes amplification of feedforward inferences that either match the predictions or contradict confident predictions, whereas the standard forms of predictive coding use feedback to convert the feedforward signal semantics into prediction errors. Furthermore, several computational studies have shown that the contextual guidance of learning and processing as formalized within the theory of coherent infomax can be directly implemented by pyramidal cells with two sites of integration, as reviewed in detail by Phillips (2015). This has not yet been done for predictive coding theories. Whether and how the strengths of the two theories can be combined in a way that builds on the evidence for AA and its regulation by adrenergic arousal remains to be seen.
There are many other important and unresolved issues. Here we list only a few, citing some relevant papers. What is the functional significance of the lower density of Ih-channels on layer 2/3 pyramidal neurons (Larkum et al., 2007)? Does it suggest that adrenergic arousal predominantly affects output from the neocortex to subcortical systems (Sheets et al., 2011), or does it arise more from biophysical than from functional differences between supragranular and infragranular cells? In what ways are the functions of Ih-channels in CA1 of the hippocampus similar to those in the neocortex, and in what ways are they different (Fan et al., 2005; Tsay et al., 2007; Shah et al., 2010; Paspalas et al., 2013; Harnett et al., 2015). Does AA make a major contribution to the evolutionary success of neocortex? How do cholinergic and dopaminergic modulation relate to adrenergic modulation via Ih-channels (Robbins and Arnsten, 2009; Arnsten et al., 2012)? Some classes of inhibitory-interneuron have evolved to produce disamplification by specifically suppressing AA (Phillips, 2015), so how do their effects interact with Ih-currents and with the dis-disamplifying effects of acetylcholine (Brombas et al., 2014)? There are strong grounds for supposing that Ih-currents and AA play a crucial role in LTP, LTD, and learning, so what exactly is that role (Brager and Johnston, 2007; Sjöström et al., 2008; Shah et al., 2010)? Our discussions of potential clinical relevance have focused predominantly on schizophrenia, but AA and its regulation by arousal is likely to play a role in several other clinical conditions (Palmer, 2014; Granato, personal communication), so how important is that role, and do the hypotheses assessed above open new possibilities for treating those conditions?
In summary, the evidence reviewed indicates that in some common classes of pyramidal cell arousal regulates apical amplification by controlling Ih-currents. This casts light on fundamental aspects of cognition, consciousness, and psychopathology, and raises many issues worthy of deeper study. If it is indeed the case that new computational primitives of amplification, disamplification, and dis-disamplification have evolved in neocortex then they will be relevant to a wide variety of cognitive functions. Studies relating AA to arousal are of particular relevance to conceptions of conscious state because they indicate that, via the adrenergic system, those primitives are turned on when we awake, and are turned up when attention is focused.
Acknowledgements
For expert comments and advice on a draft of this review we thank: Amy Arnsten, Gary Aston-Jones, Talis Bachmann, Ben Dering, John Duncan, Bruce Graham, Alberto Granato, Christiaan Levelt, Mara Mather, Kaspar Meyer, Lars Muckli, Lucy Palmer, Lucy Petro, and Barry Waterhouse. We also thank Anil Seth and two anonymous reviewers for all the hard work that they put into suggesting several ways in which an earlier version of the paper could be improved.
M.E.L. receives funding from the Human Brain Project of the European Union that includes support for his collaboration with Phillips. C.W.H. research is funded by a Natural Sciences and Engineering Research Council of Canada team grant (CH/GM). S.M.S. research is funded by National Institute of Mental Health, the New Jersey Division of Mental Health and Addiction Services, and the van Ameringen Foundation. We declare that none of this funding poses a conflict of interest for the article.
References
- Acker CD, Antic SD. Quantitative assessment of the distributions of membrane conductances involved in action potential backpropagation along basal dendrites. J Neurophysiol 2009;101: 1524–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, Mejias-Aponte CA, Clark BD. et al. Evidence for a regional specificity in the density and distribution of noradrenergic varicosities in rat cortex. J Comp Neurology 2013;521: 2195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit DJ. (1989). Modeling Brain Function. NY, New York: Cambridge University Press. [Google Scholar]
- Aoki C, Venkatesan C, Go CG. et al. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex 1998;8:269–77. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009;10:410–22. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012;76:223–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G. Behavioral functions of locus coeruleus derived from cellular attributes. Physiolo Psychol 1985;13:118–26. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005;28:403–50. [DOI] [PubMed] [Google Scholar]
- Atkinson SE, Williams SR. Postnatal development of dendritic synaptic integration in rat neocortical pyramidal neurons. J Neurophysiol 2009;102:735–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S. et al. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat Cerebral cortex. J Comp Neurol 1988;274:307–18. [DOI] [PubMed] [Google Scholar]
- Bachmann T. How a (sub)ellular coincidence detection mechanism featuring layer 5 pyramidal cells may help produce various visual phenomena. Frontiers Psychol 2015;6: doi: 10.3389/fpsyg.2015.01947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Hudetz AG. It is time to combine the two main traditions in the research on the neural correlates of consciousness: C = L × D. Frontiers Psychol 2014;5: doi:10.3389/fpsyg. 2014.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AM, Vizi ES, Zelles T. et al. α2-Adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol 2008;99:394–401. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Lüscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 2001;85: 855–68. [DOI] [PubMed] [Google Scholar]
- Berger T, Lüscher HR. Timing and precision of spike initiation in layer V pyramidal cells of the rat somatosensory cortex. Cereb Cortex 2003;13:274–81. [DOI] [PubMed] [Google Scholar]
- Berger T, Senn W, Lüscher HR. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol 2003;90: 2428–37. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S. et al. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 2009;89:847–85. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT. et al. Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet 2001;69:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 2005;28:574–82. [DOI] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through GluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci 2007;27:13926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Häusser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 2011;69:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombas A, Fletcher LN, Williams SR. Activity-dependent modulation of layer 1 inhibitory neocortical circuits by acetylcholine. J Neurosci 2014;34:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG. et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry 2005;62:1205–13. doi: 10.1001/archpsyc.62.11.1205 [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neurosci 2010;13:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, et al. α2‐Noradrenergic receptor activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol 2007;584:437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Kulics AT. The neural basis of the behaviorally relevant N1 component of the somatosensory-evoked potential in SI cortex of awake monkeys: evidence that backward cortical projections signal conscious touch sensation. Exp Brain Res 1991;84:607–19. [DOI] [PubMed] [Google Scholar]
- Chien YL, Liu CM, Shan JC, et al. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology 2015;53:1–9. doi: 10.1016/j.psyneuen.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC. et al. The DISC locus in psychiatric illness. Mol Psychiatry 2008;13:36–64. doi: 10.1038/sj.mp.4002106 [DOI] [PubMed] [Google Scholar]
- Cichon J, Gan WB. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature 2015;520:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron 2011;68:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nature Neurosci 2013;16:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH. et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nature Neurosci 2005;8:1542–51. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 1983;63:844–914. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The free-energy principle: a unified brain theory? Nat Rev Neurosci 2010;11:127–8. [DOI] [PubMed] [Google Scholar]
- Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem 2008;15:551–64. doi: 10.1101/lm.921708 [DOI] [PubMed] [Google Scholar]
- Hains AB, Yabe Y, Arnsten AF. Chronic stimulation of Alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol Stress 2015;2:1–9. doi: 10.1016/j.ynstr. 2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Xu NL, Magee JC. et al. Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 2013;79:516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Magee JC, Williams SR. Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J Neurosci 2015;35:1024–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology 2004;29:1378–85. doi: 10.1038/sj.npp.1300450 [DOI] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci 2011;12:509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S. et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA 2007;104:14501–6. doi: 10.1073/pnas. 0704774104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwy J. The neural correlates of consciousness: new experimental approaches needed? Consious Cogn 2009;18:428–38. doi:10.1016/j.concog [DOI] [PubMed] [Google Scholar]
- Huang S, Treviño M, He K. et al. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron 2012;73:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Vogt MA, Elkin H. et al. Phenotype of mice with inducible ablation of GluA1 AMPA receptors during late adolescence: relevance for mental disorders. Hippocampus 2014;24:424–35. doi: 10.1002/hipo.22236 [DOI] [PubMed] [Google Scholar]
- Jung CG. (1907). The psychology of dementia praecox In: Jung CG (ed.), Collected Works, Vol. 3—The Psychogenesis of Mental Disease. New York, NY: Nervous and Mental Disease Publ. Co., 1936. (originally published in 1907), 1–184. [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tob Res 2005;7:317–33. doi: 10.1080/1462220 0500125385 [DOI] [PubMed] [Google Scholar]
- Kay J. (1999). Neural networks for unsupervised learning based on information theory In: Kay JW, Titterington DM. (eds), Statistics and Neural Networks. New York, NY: Oxford University Press, Inc, 25–63. [Google Scholar]
- Kay J, Floreano D, Phillips WA. Contextually guided unsupervised learning using local multivariate binary processors. Neural Networks 1998;11:117–40. [DOI] [PubMed] [Google Scholar]
- Kay J, Phillips WA. Coherent infomax as a computational goal for neural systems. Bull Math Biol 2011;73:344–72. doi 10.1007/s11583-010-9564-x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. et al. Anomalous visual experiences, negative symptoms, perceptual organization and the magnocellular pathway in schizophrenia: a shared construct? Psychol Med 2005;35:1445–55. doi: S0033291705005398 [pii]10.1017/S0033291705005398 [DOI] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F. et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012;148:1051–64. doi: 10.1016/j.cell.2011.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. (2004). The Quest for Consciousness. New York: Roberts and Co. [Google Scholar]
- Koch C, Massimini M, Boly M. et al. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 2016;17:307–21. [DOI] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M. et al. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci USA 2006;103:3693–7. doi: 10.1073/pnas.0511189103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Bräuer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol 2007;578:507–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. et al. Single Ih-channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci 2006;26:1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Drew LJ. et al. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proc Natl Acad Sci USA 2011;108:E1349–58. doi: 10.1073/pnas.1114113108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 2000;23:571–9. [DOI] [PubMed] [Google Scholar]
- Larkum M. A cellular mechanism for cortical associations: an organizing principle for the Cereb. cortex. Trends Neurosci 2013;36:141–51. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M. et al. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 2009;325:756–60. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Phillips WA. Does arousal enhance apical amplification and disamplification? Behav Brain Sci 2015. (Commentary on Mather et al, Behav Brain Sci 2015;1:1–100. doi:10.1017/S0140525X15000667). [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Lüscher H-R. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex 2004;14:1059–70. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Waters J, Sakmann B. et al. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. J Neurosci 2007;27:8999–9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 1999;98:338–41. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol (Lond) 2001;533:447–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledergerber D, Larkum ME. Properties of layer 6 pyramidal neuron dendrites. J Neurosci 2010;30:13031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BM, Mao ZM, Wang M. et al. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology 1999;21:601–10. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol 1994;62:134–9. [DOI] [PubMed] [Google Scholar]
- Lörincz A, Notomi T, Tamas G. et al. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 2002;5:1185–93. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 1999;2:508–14. [DOI] [PubMed] [Google Scholar]
- Major G, Larkum ME, Schiller J. Active properties of neocortical pyramidal neuron dendrites. Annu Rev Neurosci 2013;36:1–24. [DOI] [PubMed] [Google Scholar]
- Manita S, Suzuki T, Homma C. et al. A top-down cortical circuit for accurate sensory perception. Neuron 2015;86:1304–16. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M. et al. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci 2015;1:1–100. doi:10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley MJ, Vinck M, Reimer J. et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron 2015;87:1143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. The role of dendritic signaling in the anesthetic suppression of consciousness. Anesthesiology 2015;122:1415–31. [DOI] [PubMed] [Google Scholar]
- Millar JK, Mackie S, Clapcote SJ. et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol 2007;584:401–5. doi: 10.1113/jphysiol. 2007.140210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S. et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005;310:1187–91. doi: 10.1126/science.1112915 [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S. et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 2000;9:1415–23. [DOI] [PubMed] [Google Scholar]
- Minor KS, Marggraf MP, Davis BJ. et al. Conceptual disorganization weakens links in cognitive pathways: disentangling neurocognition, social cognition, and metacognition in schizophrenia. Schizophr Res 2015; doi: 10.1016/j.schres.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Muckli L, De Martino F, Vizioli L. et al. Contextual feedback to superficial layers of V1. Curr Biol 2015;25:2690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Pérez-Garci E, Nevian T. et al. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 2009;457:1137–41. [DOI] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A. et al. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci 2007;10:206–14. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT. et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 2004;119:719–32. [DOI] [PubMed] [Google Scholar]
- Oizumi M, Albantakis L, Tononi G. From the phenomenology to the mechanisms of consciousness: integrated information theory 3.0. PLoS Comput Biol 2014;10:e1003588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM. Dendritic integration in pyramidal neurons during network activity and disease. Brain Res Bull 2014;103:2–10. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Shai AS, Reeve JE. et al. NMDA spikes enhance action potential generation during sensory input. Nat Neurosci 2014;17:383–90. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Wang M, Arnsten AFT. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia. Cereb Cortex 2013;23: 1643–54. 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA. Cognitive functions of intracellular mechanisms for contextual amplification. Brain Cogn 2015. 10.1016/j.bandc.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Clark A, Silverstein SM. On the functions, mechanisms, and malfunctions of intracortical contextual modulation. Neurosci Biobehav Rev 2015;52:1–20. doi:10.1016/j.neubiorev.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Phillips W, Kay J, Smyth D. The discovery of structure by multi-stream networks of local processors with contextual guidance. Network: Comput Neural Systems 1995;6:225–46. [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci 2003;26:65–82. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. The coherent organization of mental life depends on mechanisms for context-sensitive gain-control that are impaired in schizophrenia. Front Psychol 2013;4:307. doi: 10.3389/fpsyg.2013.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Singer W. In search of common foundations for cortical computation. Behav. Brain Sci 1997;20:657–83. discussion 683–722. [DOI] [PubMed] [Google Scholar]
- Phillips WA, von der Malsburg C, Singer W. Dynamic coordination in brain and mind In: von der Malsburg C, Phillips WA, Singer W.(eds), Dynamic Coordination in the Brain: From Neurons to Mind, Cambridge, MA: MIT Press, 2010. [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O. et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 2008;13:173–86. 115. doi: 10.1038/sj.mp.4002079 [DOI] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 2013;16:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E. et al. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 2004;92:361–71. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Colgan L, Nou E, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry 2005;58:894–900. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 2009;32:267–87. doi: 10.1146/annurev.neuro. 051508.135535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaai H, Neves R, Eschenko O, et al. Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc Natl Acad Sci USA 2015;112:12834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Köhr G, Treviño M. Noradrenergic ‘tone’ determines dichotomous control of cortical spike-timing-dependent plasticity. Scientific Reports 2012;2:417. doi: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarone S, Manzone L, Gambini O, et al. The dream as a model for psychosis: an experimental approach using bizarreness as a cognitive marker. Schiz Bull 2008;34:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013;17:565–73. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psych 2011;2:395.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci 2010;33:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Suter BA, Kiritani T. et al. Corticospinal-specific HCN expression in mouse motor cortex: Ih-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J Neurophysiol 2011;106:2216–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Berten S, Essex B, et al. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci 2009;8:175–202. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A. et al. Dendritic excitability and synaptic plasticity. Physiol Rev 2008;88:769–840. [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W. et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 1990;336:13–36. [DOI] [PubMed] [Google Scholar]
- Sterley TL, Howells FM, Russell VA. Nicotine-stimulated release of [3H]norepinephrine is reduced in the hippocampus of an animal model of attention-deficit/hyperactivity disorder, the spontaneously hypertensive rat. Brain Res 2014;1572:1–10. doi: 10.1016/j.brainres.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality Soc Psychol Rev 2004;8:220–47. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Larkum ME. (2016). Dendritic dynamics in sensory perception. Poster presented at 10th FENS Forum of Neuroscience, Copenhagen.
- Torniainen M, Suvisaari J, Partonen T. et al. Cognitive impairments in schizophrenia and schizoaffective disorder: relationship with clinical characteristics. J Nerv Ment Dis 2012; 200:316–22. doi: 10.1097/NMD.0b013e31824cb359 [DOI] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2_ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 2007;56:1076–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman S. Sequence seeking and counter streams: a computational model for bidirectional information flow in the visual cortex. Cereb Cortex 1995;5:1–11. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci USA 2014;111: 3859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG. et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 2007;10:376–84. doi: 10.1038/nn1846 [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD. et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S, 3R-ACPD. J Neurosci 1993;13:2199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibral M, Priesemann V, Kay JW. et al. Partial information decomposition as a unified approach to the specification of neural goal functions. Brain Cogn 2015. 10.1016/j.bandc.2015.004 [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol 2000;83:3177–82. [DOI] [PubMed] [Google Scholar]
- Xu NL, Harnett MT, Williams SR, et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 2012;492:247–51. [DOI] [PubMed] [Google Scholar]
- Zhou C, Liu J, Chen XD. General anesthesia mediated by effects on ion channels. World J Critical Care Medicine 2012;1:80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol 2000;526:571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]